Claim Denials for Cancer-Related Next-Generation Sequencing in Medicare
- PMID: 40249617
- PMCID: PMC12008754
- DOI: 10.1001/jamanetworkopen.2025.5785
Claim Denials for Cancer-Related Next-Generation Sequencing in Medicare
Abstract
Importance: The Centers for Medicare & Medicaid Services implemented a National Coverage Determination (NCD) for genetic testing using next-generation sequencing (NGS) in March 2018 and amended it in January 2020. Little is known about how often NGS insurance claims are denied and what factors are associated with these denials.
Objective: To examine the prevalence of and factors associated with claim denials for NGS testing among Medicare enrollees.
Design, setting, and participants: This cohort study used a 20% random sample of Medicare claims data from January 1, 2016, to December 31, 2021. Statistical analysis took place from June to October 2024.
Main outcomes and measures: Claim denials were measured based on payment and denial data. Multivariable logistic regression models were estimated with patient and claim characteristics, including testing type, testing site, and time period indicators reflecting the presence and scope of the NCD: no NCD (period 1), first NCD (period 2), and amended NCD (period 3).
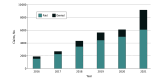
Results: The study sample included 29 919 cancer-related NGS claims among 24 443 unique Medicare beneficiaries (51.8% males; 44.7% aged >75 years). The number of cancer-related NGS testing claims increased from 1912 in 2016 to 9177 in 2021. Claim denial occurred among 23.3% of the NGS claims in the sample. The claim denial rate was 16.8% before the NCD, 20.3% after the implementation of the NCD in 2018, and 27.4% after the amended NCD in 2020. Claims for NGS testing were more likely to be denied if they were performed in independent laboratories (odds ratio [OR], 2.76 [95% CI, 2.58-2.95]; P < .001) or other nonhospital sites (OR, 2.55 [95% CI, 2.12-3.07]; P < .001) vs a hospital or if they were for 50 or more genes vs 50 or fewer genes for solid tumors (OR, 1.32 [95% CI, 1.23-1.43]; P < .001). The likelihood of a claim denial was higher in both time periods after the original 2018 NCD compared with before the NCD (period 2: OR, 1.23 [95% CI, 1.10-1.36]; P < .001; period 3: OR, 1.64 [95% CI, 1.49-1.80]; P < .001). The claim denial likelihood for period 3 was significantly higher than period 2 (OR, 1.34 [95% CI, 1.24-1.44]; P < .001).
Conclusions and relevance: In this cohort study of cancer-related NGS testing claims among Medicare enrollees, denial rates varied by testing type and testing site and increased over time. The findings suggest the continued existence of uncertainty regarding coverage for NGS, despite the NCD. Policy approaches to further reduce uncertainty regarding NGS coverage and raise awareness of potential financial liability warrant consideration.
Conflict of interest statement
Figures
Similar articles
-
Are Quality Scores in the Centers for Medicaid and Medicare Services Merit-based Incentive Payment System Associated With Outcomes After Outpatient Orthopaedic Surgery?Clin Orthop Relat Res. 2024 Jul 1;482(7):1107-1116. doi: 10.1097/CORR.0000000000003033. Epub 2024 Mar 21. Clin Orthop Relat Res. 2024. PMID: 38513092 Free PMC article.
-
Use of Multiplex Molecular Panels to Diagnose Urinary Tract Infection in Older Adults.JAMA Netw Open. 2024 Nov 4;7(11):e2446842. doi: 10.1001/jamanetworkopen.2024.46842. JAMA Netw Open. 2024. PMID: 39589743 Free PMC article.
-
Trends in Use of Next-Generation Sequencing in Patients With Solid Tumors by Race and Ethnicity After Implementation of the Medicare National Coverage Determination.JAMA Netw Open. 2021 Dec 1;4(12):e2138219. doi: 10.1001/jamanetworkopen.2021.38219. JAMA Netw Open. 2021. PMID: 34882180 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
Cited by
-
Metagenomic Next-Generation Sequencing in Infectious Diseases: Clinical Applications, Translational Challenges, and Future Directions.Diagnostics (Basel). 2025 Aug 8;15(16):1991. doi: 10.3390/diagnostics15161991. Diagnostics (Basel). 2025. PMID: 40870843 Free PMC article. Review.
References
-
- Data brief: Medicare Part B spending on lab tests increased in 2021, driven by higher volume of COVID-19 tests, genetic tests, and chemistry tests. U.S. Department of Health and Human Services, Office of Inspector General. Published December 2022. Accessed January 31, 2025. https://oig.hhs.gov/oei/reports/OEI-09-22-00400.pdf
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical