Molecular basis of pyruvate transport and inhibition of the human mitochondrial pyruvate carrier
- PMID: 40249800
- PMCID: PMC12007569
- DOI: 10.1126/sciadv.adw1489
Molecular basis of pyruvate transport and inhibition of the human mitochondrial pyruvate carrier
Abstract
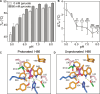
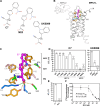
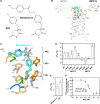
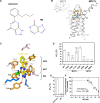
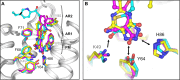
The mitochondrial pyruvate carrier transports pyruvate, produced by glycolysis from sugar molecules, into the mitochondrial matrix, as a crucial transport step in eukaryotic energy metabolism. The carrier is a drug target for the treatment of cancers, diabetes mellitus, neurodegeneration, and metabolic dysfunction-associated steatotic liver disease. We have solved the structure of the human MPC1L/MPC2 heterodimer in the inward- and outward-open states by cryo-electron microscopy, revealing its alternating access rocker-switch mechanism. The carrier has a central binding site for pyruvate, which contains an essential lysine and histidine residue, important for its ΔpH-dependent transport mechanism. We have also determined the binding poses of three chemically distinct inhibitor classes, which exploit the same binding site in the outward-open state by mimicking pyruvate interactions and by using aromatic stacking interactions.
Figures




References
-
- Bricker D. K., Taylor E. B., Schell J. C., Orsak T., Boutron A., Chen Y. C., Cox J. E., Cardon C. M., Van Vranken J. G., Dephoure N., Redin C., Boudina S., Gygi S. P., Brivet M., Thummel C. S., Rutter J., A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 (2012). - PMC - PubMed
-
- Bolsterli B. K., Boltshauser E., Palmieri L., Spenger J., Brunner-Krainz M., Distelmaier F., Freisinger P., Geis T., Gropman A. L., Haberle J., Hentschel J., Jeandidier B., Karall D., Keren B., Klabunde-Cherwon A., Konstantopoulou V., Kottke R., Lasorsa F. M., Makowski C., Mignot C., O'Gorman Tuura R., Porcelli V., Santer R., Sen K., Steinbrucker K., Syrbe S., Wagner M., Ziegler A., Zoggeler T., Mayr J. A., Prokisch H., Wortmann S. B., Ketogenic diet treatment of defects in the mitochondrial malate aspartate shuttle and pyruvate carrier. Nutrients 14, 3605 (2022). - PMC - PubMed
-
- Klingenberg M., Mitochondria metabolite transport. FEBS Lett. 6, 145–154 (1970). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

