Hippocampal perineuronal net degradation identifies prefrontal and striatal circuits involved in schizophrenia-like changes in marmosets
- PMID: 40249814
- PMCID: PMC12007587
- DOI: 10.1126/sciadv.adu0975
Hippocampal perineuronal net degradation identifies prefrontal and striatal circuits involved in schizophrenia-like changes in marmosets
Abstract
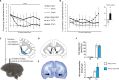
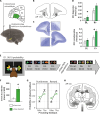
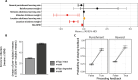
In schizophrenia, anterior hippocampus (aHipp) overactivity is associated with orbitofrontal cortex (OFC) dysfunction, but the contribution to symptomatology is unknown. In rodents, degradation of the hippocampal perineuronal net (PNN) replicates this overactivity, but uncertainty over rodent/human prefrontal homology limits translation to humans. Here, we test the hypothesis that aHipp PNN degradation in a species with a human-like prefrontal cortex, the marmoset, alters aHipp-striatal and aHipp-OFC circuitry. Microdialysis and [18F]-fluoro-l-dihydroxyphenylalanine positron emission tomography identified increased dopamine synthesis in the associative striatum, but not the nucleus accumbens, as is seen in schizophrenia, and elevated dopamine and noradrenaline in the OFC. Behaviorally, activity was elevated in a marmoset version of the amphetamine-induced activity test, and impaired probabilistic discrimination learning was seen in an OFC/striatum-dependent task that computational modeling suggests was due to loss of goal-directed behavior. Together, these findings demonstrate that a loss of primate aHipp PNNs is sufficient to induce striatal and prefrontal dysfunction consistent with that observed in humans with schizophrenia.
Figures


References
-
- Schobel S. A., Chaudhury N. H., Khan U. A., Paniagua B., Styner M. A., Asllani I., Inbar B. P., Corcoran C. M., Lieberman J. A., Moore H., Small S. A., Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93 (2013). - PMC - PubMed
-
- Kaar S. J., Natesan S., McCutcheon R., Howes O. D., Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 172, 107704 (2020). - PubMed
-
- Shurman B., Horan W. P., Nuechterlein K. H., Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr. Res. 72, 215–224 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

