Atezolizumab consolidation in patients with high-risk diffuse large B-cell lymphoma in complete remission after R-CHOP
- PMID: 40249860
- PMCID: PMC12274673
- DOI: 10.1182/bloodadvances.2024015226
Atezolizumab consolidation in patients with high-risk diffuse large B-cell lymphoma in complete remission after R-CHOP
Abstract
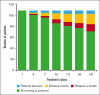
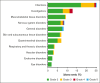
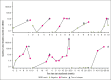
The risk of relapse among high-risk patients with diffuse large B-cell lymphoma (DLBCL) in complete metabolic remission (CMR) after rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) therapy is 20% to 25%. Here, we evaluated whether consolidation with the programmed cell death ligand 1 checkpoint inhibitor atezolizumab could reduce the relapse risk. In this phase 2, open-label trial, patients with DLBCL with an International Prognostic Index (IPI) score of ≥3 and CMR after R-CHOP received 1200 mg atezolizumab every 3 weeks for 18 cycles. The primary end point was disease-free survival (DFS) at 2 years, with the aim of improving it to 89% compared to historical 79%. Secondary end points included overall survival (OS) and safety (Common Terminology Criteria for Adverse Events version 4.0). Analyses were on an intention-to-treat principle. Of 109 patients, 65% completed treatment. The cohort was 59% males, with 63% having high-intermediate risk IPI scores. At a median follow-up of 36.4 months, 15 relapses occurred (median, 8.2 months). The 2-year DFS was 87.9% (90% confidence interval [CI], 81.5-92.1), and the 2-year OS was 96.3% (90% CI, 91.7-98.3), meeting the primary objective. Treatment with salvage chemotherapy resulted in 10 of 13 patients achieving a second CMR. OS was significantly better among atezolizumab-treated patients than in a population-based matched control cohort from the Netherlands Cancer Registry. Adverse events (AEs) affected 79% of patients, with 18% developing immune-related AEs, including 4.5% grade 3 to 4. Atezolizumab consolidation significantly improved DFS in high-risk patients with DLBCL compared to historical cohorts. OS was significantly better than a population-based control cohort. These findings warrant further validation and assessment of immune checkpoint inhibitors as consolidation strategy in DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT03463057.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.N. reports research support from Takeda; travel support from AbbVie; and participation in data safety monitoring board or advisory board for AbbVie. M.C. declares research support from Bristol Myers Squibb, Gilead, and Genmab, and participation on advisory boards for AbbVie, Novartis, and Incyte. The remaining authors declare no competing financial interests.
Figures



References
-
- Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. - PubMed
-
- El-Galaly TC, Jakobsen LH, Hutchings M, et al. Routine imaging for diffuse large B-cell lymphoma in first complete remission does not improve post-treatment survival: a Danish-Swedish population-based study. J Clin Oncol. 2015;33(34):3993–3998. - PubMed
-
- Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol. 2018;29(3):707–714. - PubMed
-
- Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473–2481. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

