The OHI index predicts early mortality from organ dysfunction and survival benefit from etoposide in patients with lymphoma
- PMID: 40249910
- PMCID: PMC12447441
- DOI: 10.1182/bloodadvances.2024014005
The OHI index predicts early mortality from organ dysfunction and survival benefit from etoposide in patients with lymphoma
Abstract
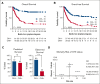
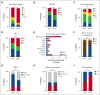
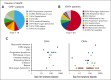
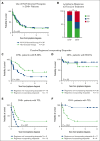
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome that complicates hematologic malignancies. The Optimized HLH Inflammatory (OHI) index, based solely on the combined elevation of soluble CD25 (sCD25; >3900 U/mL) and ferritin (1000 ng/mL) levels, predicts mortality more effectively than the conventional HLH criteria. This study aimed to determine whether mortality in OHI-positive (OHI+) patients is primarily caused by lymphoma or inflammation-related causes. In a multicenter, retrospective study of patients with newly diagnosed lymphoma with sCD25 and ferritin measurements available, patients were classified as OHI+ or OHI negative (OHI-). The 1-year and 3-year overall survival (OS), event-free survival, and causes of death were analyzed, along with predicted vs observed mortality based on other lymphoma-relevant prognostic indexes. Among 135 patients with lymphoma (70 OHI- and 65 OHI+), OHI+ patients had significantly lower OS at 1 year (33% vs 81%; P < .0001) with a median survival of 190 days. OHI+ patients had a 13-fold increased mortality risk (odds ratio, 13.3; 95% confidence interval, 6.0-28.5) despite similar predicted OS based on conventional indexes (72% vs 65%; P = .46). Mortality causes differed significantly. A total of 58% of OHI+ patients died from multiorgan failure, whereas only 6% died from progressive lymphoma. In contrast, 43% of the OHI- patients died from lymphoma progression. The incorporation of etoposide into lymphoma-directed treatment was associated with improved OS in OHI+ T-cell lymphomas (P = .007). These findings underscore the clinical significance of the OHI index as a prognostic tool in lymphoma, to elucidate the mechanisms of mortality, and to identify a high-risk subgroup for which tailored treatments may lead to improved outcomes.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.Z.-L. and M.B.J. report consulting fees from Sobi. R.G. reports consulting fees from Roche, Takeda, Novartis, Gilead Sciences, and AbbVie, none of which is directly related to the content of this study. J.M. reports consulting fees from Chugai Pharmaceutical for work that was not directly related to the content of this article. The remaining authors declare no competing financial interests.
Figures

Comment in
-
"Optimizing" HLH diagnoses in patients with lymphoma.Blood Adv. 2025 Sep 9;9(17):4513-4514. doi: 10.1182/bloodadvances.2025017104. Blood Adv. 2025. PMID: 40924432 Free PMC article. No abstract available.
References
-
- Setiadi A, Zoref-Lorenz A, Lee CY, Jordan MB, Chen LYC. Malignancy-associated haemophagocytic lymphohistiocytosis. Lancet Haematol. 2022;9(3):e217–e227. - PubMed
-
- La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. - PubMed
-
- Ishii E, Ohga S, Imashuku S, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

