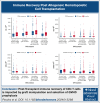
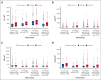
Delayed T-cell recovery after hematopoietic cell transplantation is associated with decreased overall survival in adults
- PMID: 40249911
- PMCID: PMC12274672
- DOI: 10.1182/bloodadvances.2024015288
Delayed T-cell recovery after hematopoietic cell transplantation is associated with decreased overall survival in adults
Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) can provide curative treatment for hematologic malignancies but is associated with prolonged lymphopenia that may contribute to an increased risk of infection and relapse, resulting in decreased survival. We hypothesized that patients with rapid and robust CD4 T- and B-cell recovery have improved survival and decreased treatment-related mortality (TRM). A total of 2089 patients were included who underwent first allo-HCT for acute myeloid leukemia/acute lymphoblastic leukemia/myelodysplastic syndrome from 2008 to 2019 reported to the Center for International Blood and Marrow Transplant Research with available CD4 counts at days 100 and 180. Patients (median age, 51 years [range, 2-75]) were categorized into 4 groups based on graft-versus-host disease (GVHD) prophylaxis: ex vivo T-cell depletion (TCD/CD34), posttransplant cyclophosphamide, calcineurin inhibitor alone (CNI), or CNI with antithymocyte globulin. Based upon survival, we could identify optimal cutoff points for CD4+ T cells in pediatric (age of <20 years) patients: 248 × 106/L and 420 × 106/L at days 100 and 180, respectively; and in adult (age of >20 years) patients: 104 × 106/L and 115 × 106/L at days 100 and 180, respectively. In adults, day-100 CD4 count was associated with overall survival (OS), progression-free survival (PFS), and TRM but not relapse, incidence of infections, or chronic GVHD. Similarly, CD4 counts above the cutoff point at day 180 in adults were associated with improved OS, PFS, and TRM but no other outcomes. No clinical associations for CD4 counts were identifiable in pediatric patients. These findings underscore the importance of tailoring transplant strategies for adults to optimize immune recovery and improve patient outcomes.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.-A.P. reports honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on data safety monitoring boards for Cidara Therapeutics and SELLAS Life Sciences; serves on the scientific advisory board of NexImmune; has ownership interests in NexImmune, Omeros, and Orca Bio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. P.S. serves as an ad hoc consultant to Forge Bio. M.R. reports compensation as an employee (executive director, clinical development) of Kura Oncology and reports equity stock options as an employee of Kura Oncology. R.F.C. reports compensation from ADMA Biologics, Janssen, MSD/Merck, Roche, Takeda Pharmaceuticals, Shionogi, Genentech, Astellas Pharma, AiCuris, Adagio Therapeutics, Oxford Immunotec, Karius, Moderna, and Ansun Pharmaceuticals, and significant payments from Merck, Karius, Aicuris, Ansun Pharmaceuticals, Takeda Pharmaceuticals, Genentech, Oxford Immunotec, and Eurofins Viracor. J.A.H. reports compensation for consulting roles from AlloVir, EVERSANA, GeoVax, Grifols, Karius, Modulus, Senti Bio, Takeda Pharmaceuticals, and UpToDate; serves on a data safety monitoring board for Moderna; reports compensation for speaking from Gilead Australia; reports research support from AlloVir, Deverra, GeoVax, Merck, Oxford Immunotec, and Takeda Pharmaceuticals; and received significant payments for participation on advisory board meeting from Takeda Pharmaceuticals. The remaining authors declare no comping financial interests.
Figures
Similar articles
-
Blinatumomab Therapy Is Associated with Favorable Outcomes after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients with B Cell Acute Lymphoblastic Leukemia.Transplant Cell Ther. 2024 Feb;30(2):217-227. doi: 10.1016/j.jtct.2023.10.024. Epub 2023 Nov 4. Transplant Cell Ther. 2024. PMID: 37931800
-
Incidence and Impact of Fungal Infections in Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis and Haploidentical Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Analysis.Transplant Cell Ther. 2024 Jan;30(1):114.e1-114.e16. doi: 10.1016/j.jtct.2023.09.017. Epub 2023 Sep 28. Transplant Cell Ther. 2024. PMID: 37775070 Free PMC article.
-
Mycophenolate mofetil versus methotrexate for prevention of graft-versus-host disease in people receiving allogeneic hematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2014 Jul 25;2014(7):CD010280. doi: 10.1002/14651858.CD010280.pub2. Cochrane Database Syst Rev. 2014. PMID: 25061777 Free PMC article.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2014 Apr 20;2014(4):CD010189. doi: 10.1002/14651858.CD010189.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Nov 7;11:CD010189. doi: 10.1002/14651858.CD010189.pub3. PMID: 24748537 Free PMC article. Updated.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2024 Nov 7;11(11):CD010189. doi: 10.1002/14651858.CD010189.pub3. Cochrane Database Syst Rev. 2024. PMID: 39508306
References
-
- Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–480. - PubMed
-
- Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002;73(7):1154–1158. - PubMed
-
- Storek J, Viganego F, Dawson MA, et al. Factors affecting antibody levels after allogeneic hematopoietic cell transplantation. Blood. 2003;101(8):3319–3324. - PubMed
-
- Small TN, Avigan D, Dupont B, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transpl. 1997;3(2):65–75. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials