Mitochondrial Imbalance in Down Syndrome: A Driver of Accelerated Brain Aging?
- PMID: 40249934
- PMCID: PMC12339184
- DOI: 10.14336/AD.2025.0189
Mitochondrial Imbalance in Down Syndrome: A Driver of Accelerated Brain Aging?
Abstract
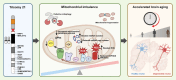
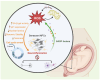
Down syndrome (DS), caused by trisomy of chromosome 21 (HSA21), is a complex condition associated with neurodevelopmental impairments and accelerated brain aging, often culminating in early-onset Alzheimer's disease (AD). Central to this accelerated aging is mitochondrial imbalance, characterized by disrupted energy metabolism, increased oxidative stress, impaired dynamics, and defective quality control mechanisms like mitophagy. These abnormalities exacerbate neuronal vulnerability, driving cognitive decline and neurodegeneration. This review examines the genetic and biochemical underpinnings of mitochondrial dysfunction in DS, with a focus on the role of HSA21-encoded genes. We also highlight how mitochondrial dysfunction, amplified by oxidative stress and HSA21 gene dosage effects, converges with cellular senescence and neuroinflammation to accelerate Alzheimer-like pathology and brain aging in DS. Finally, we discuss emerging therapeutic strategies targeting mitochondrial pathways, which hold promise for mitigating neurodegenerative phenotypes and improving outcomes in DS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Bustamante-Barrientos FA, Luque-Campos N, Araya MJ, Lara-Barba E, de Solminihac J, Pradenas C, et al. (2023). Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J Transl Med, 21:613. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
