Regional comparison of efficacy and safety for vilobelimab in critically ill, invasively mechanically ventilated COVID-19 patients
- PMID: 40250846
- PMCID: PMC12010313
- DOI: 10.1136/bmjresp-2023-002206
Regional comparison of efficacy and safety for vilobelimab in critically ill, invasively mechanically ventilated COVID-19 patients
Abstract
Background: Vilobelimab, a first in class C5a-specific monoclonal antibody, improved 28-day and 60-day mortality in intubated COVID-19 patients in PANAMO, a phase 3 randomised, double-blind, placebo-controlled multicentre study. All-cause mortality was pre-specified to be analysed pooling by region (western Europe, South America, South Africa/Russia).
Methods: Critically ill, invasively mechanically ventilated COVID-19 patients were randomised in a 1:1 ratio within 48 hours of intubation to receive vilobelimab treatment (six, 800 mg intravenous infusions) or placebo on top of standard of care. We analysed the efficacy and safety of vilobelimab based on prespecified geographic regions.
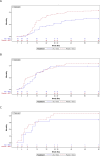
Results: 368 patients were randomised and analysed: 177 in the vilobelimab group and 191 in the placebo group. In western Europe (n=209), 28-day all-cause mortality was significantly lower in the vilobelimab group (21%) compared with placebo (37%) (HR 0.51 (95% CI: 0.30, 0.87), p=0.014). In South America (n=126), mortality was similar between groups (40% vs 37%; HR 0.94 (95% CI: 0.53, 1.67), p=0.83). In South Africa/Russia (n=33), mortality was 69% in the vilobelimab group and 87% in the placebo group (HR 0.62 (95% CI: 0.28, 1.38), p=0.25). Within the Brazilian subpopulation (n=74), a significant age imbalance between the vilobelimab and placebo group was detected (median 53.5 years in the vilobelimab group vs 44.5 years in the placebo group). Occurrence of treatment-emergent adverse events between regions was similar.
Conclusion: The most apparent 28-day all-cause mortality benefit for vilobelimab was in western Europe. Age imbalance between treatment groups in Brazil may have resulted in a lower efficacy signal for vilobelimab in South America compared with other regions. Overall, vilobelimab demonstrated a favourable safety profile and reduced mortality in critically ill, intubated COVID-19 patients, with regional variations influencing outcomes.
Keywords: ARDS; COVID-19; Critical Care; Innate Immunity; Respiratory Infection; Viral infection.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SR is an employee of Metronomia Clinical Research, a contracted statistical service provider for InflaRx. CT, BPB, RZ and CC are employees of InflaRx and may hold shares and/or stock options in InflaRx. NCR and RG are founders, active officers, and executive directors of the board, and hold shares and stock options in InflaRx. APJV received consulting fees from InflaRx for advisory work, paid to the institution. All other authors declare no competing interests.
Figures



References
-
- World Health Organization Statement on the fifteenth meeting of the IHR. 2005. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet... Available.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
