Dietary addition of magnesium hydride nanoparticles: a breakthrough in combating high-fat diet-induced chronic kidney disease
- PMID: 40251018
- PMCID: PMC12054669
- DOI: 10.4103/mgr.MEDGASRES-D-24-00090
Dietary addition of magnesium hydride nanoparticles: a breakthrough in combating high-fat diet-induced chronic kidney disease
Abstract
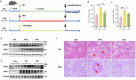
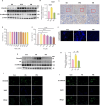
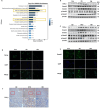
A substantial body of evidence indicates a positive correlation between dyslipidemia and an elevated risk of chronic kidney disease, with renal interstitial fibrosis frequently serving as a common pathway in the advanced stages of chronic kidney disease progression. Hydrogen has anti-inflammatory and antioxidant properties, and magnesium hydride nanoparticle is a material with high hydrogen storage capacity. Magnesium hydride -fortified feed is capable of releasing hydrogen gas steadily and continuously within the digestive tract. A 12-week high-fat diet significantly elevated the serum urea and creatinine levels in mice. In contrast, dietary addition of magnesium hydride demonstrated a notable protective effect against pathological conditions. Additionally, magnesium hydride -fortified feed was found to reduce renal fibrosis and thereby improve renal function. In support of these findings, an in vitro study utilizing human kidney cortical proximal tubule epithelial cells (HK-2 cells) exposed to palmitic acid under conditions mimicking a high-fat diet confirmed the renoprotective effects of magnesium hydride. Furthermore, the primary target phosphatase and tensin homologue deleted on chromosome 10 and the molecular mechanisms underlying the effects of magnesium hydride, specifically its ability to inhibit the transforming growth factor-beta -Smad family member 2 and 3 (Smad2/3) axis through downregulating the expression of phosphatase and tensin homologue deleted on chromosome 10, were elucidated. Additionally, overexpression of Hes family BHLH transcription factor 1 can negate the beneficial effects of magnesium hydride, suggesting that Hes family BHLH transcription factor 1 may serve as an upstream regulatory target in the context of the effects of magnesium hydride. In conclusion, this study demonstrated that magnesium hydride functions as a safe and effective hydrogen source capable of inhibiting the activation of the transforming growth factor-beta/Smad2/3 and protein kinase B/mechanistic target of rapamycin pathways by increasing the expression of phosphatase and tensin homologue deleted on chromosome 10. This mechanism counteracts the progression of high-fat diet-induced chronic renal damage.
Keywords: chronic kidney disease; high-fat diet; magnesium hydride; phosphatase and tensin homolog deleted on chromosome ten; renal fibrosis.
Copyright © 2025 Medical Gas Research.
Conflict of interest statement
Figures


Similar articles
-
Ambient particulate matter exposure plus a high-fat diet exacerbate renal injury by activating the NLRP3 inflammasome and TGF-β1/Smad2 signaling pathway in mice.Ecotoxicol Environ Saf. 2022 Jun 15;238:113571. doi: 10.1016/j.ecoenv.2022.113571. Epub 2022 May 2. Ecotoxicol Environ Saf. 2022. PMID: 35512472
-
Excessive dietary lipid intake provokes an acquired form of lysosomal lipid storage disease in the kidney.J Pathol. 2018 Dec;246(4):470-484. doi: 10.1002/path.5150. Epub 2018 Nov 5. J Pathol. 2018. PMID: 30073645
-
Silymarin protects against renal injury through normalization of lipid metabolism and mitochondrial biogenesis in high fat-fed mice.Free Radic Biol Med. 2017 Sep;110:240-249. doi: 10.1016/j.freeradbiomed.2017.06.009. Epub 2017 Jun 15. Free Radic Biol Med. 2017. PMID: 28625483
-
Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities.Clin Sci (Lond). 2021 Jan 29;135(2):275-303. doi: 10.1042/CS20201213. Clin Sci (Lond). 2021. PMID: 33480423 Review.
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
References
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. - PubMed
-
- Mladenović D, Vesković M, Šutulović N, et al. Adipose-derived extracellular vesicles - a novel cross-talk mechanism in insulin resistance, nonalcoholic fatty liver disease, and polycystic ovary syndrome. Endocrine. 2024;85:18–34. - PubMed
-
- Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. - PubMed
-
- Xu C, Hong Q, Zhuang K, et al. Regulation of pericyte metabolic reprogramming restricts the AKI to CKD transition. Metabolism. 2023;145:155592. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

