Determining potential immunomodulatory drug efficacy in sepsis using ELISpot
- PMID: 40251188
- PMCID: PMC12008245
- DOI: 10.1038/s41598-025-92016-6
Determining potential immunomodulatory drug efficacy in sepsis using ELISpot
Abstract
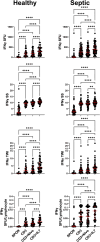
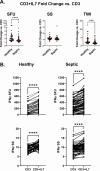
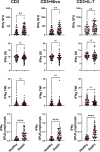
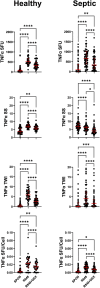
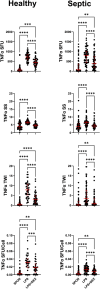
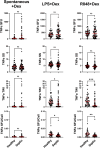
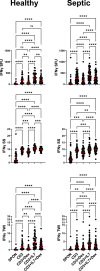
This study evaluated the ability of ELISpot to identify potential immuno-modulatory drug therapies in sepsis. ELISpot was performed ex vivo on whole blood from septic patients and healthy controls. Innate and adaptive immunity were evaluated by production of TNF-α and IFN-γ, respectively. Drug efficacy was determined by their effects to modulate the both the number of cytokine-producing cells and amount of cytokine produced per cell. The corticosteroid dexamethasone was evaluated for its ability to down modulate TNF-α and IFN-γ production. The TLR7/8 agonist resiquimod (R848) and T cell stimulants IL-7 and anti-PD-1 mAb were tested for their ability to enhance immunity. LPS and resiquimod increased total TNF-α production in septic patients by 1,549% and 1,829%, respectively. Conversely, dexamethasone diminished the responses to LPS or resiquimod by 75% and 61%, respectively. IL-7, but not anti-PD-1 mAb markedly increased IFN-γ production in both healthy subjects (121%) and septic patients (82%). Dexamethasone also reduced anti-CD3/CD28 mAb stimulated IFN-γ production by 69%; while IL-7 ameliorated dexamethasone-induced suppression. IL-7 significantly enhanced lymphocyte function in over 90% of septic patients. ELISpot can reveal host immune response patterns and the effects of drugs to selectively down- or up-regulate patient immunity. Furthermore, the ability of ELISpot to detect the effect of specific immuno-modulatory drugs to independently regulate the innate and adaptive host response could enable precision-based immune drug therapies in sepsis.
Keywords: Adaptive immunity; Anti-PD-1; Checkpoint inhibitors; Corticosteroids; IL-7; Innate immunity.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Competing interests: MBM, and KER are members of Immune Functional Diagnostics, LLC and receive no direct financial compensation. Immune Functional Diagnostics, LLC is developing predictive metrics in critical illness and this technology (provisional patent application 63/521,817) is evaluated in this research. SCB, LLM, RSH, and the University of Florida may receive royalty income based on a technology developed by SCB and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research. CCC and the University of Cincinnati may receive royalty income based on a technology developed by CCC and others and licensed by Washington University in St. Louis to IFDx LLC. That technology is evaluated in this research.
Figures
Similar articles
-
TEMPORAL CHANGES IN INNATE AND ADAPTIVE IMMUNITY DURING SEPSIS AS DETERMINED BY ELISPOT.Shock. 2024 Aug 1;62(2):255-264. doi: 10.1097/SHK.0000000000002377. Epub 2024 May 3. Shock. 2024. PMID: 38754032 Free PMC article.
-
Temporal Changes in Innate and Adaptive Immunity During Sepsis as Determined by ELISpot.bioRxiv [Preprint]. 2023 Dec 14:2023.12.14.571668. doi: 10.1101/2023.12.14.571668. bioRxiv. 2023. Update in: Shock. 2024 Aug 1;62(2):255-264. doi: 10.1097/SHK.0000000000002377. PMID: 38168302 Free PMC article. Updated. Preprint.
-
Surviving septic patients endotyped with a functional assay demonstrate active immune responses.Front Immunol. 2024 Oct 14;15:1418613. doi: 10.3389/fimmu.2024.1418613. eCollection 2024. Front Immunol. 2024. PMID: 39469706 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy.Cochrane Database Syst Rev. 2017 May 8;5(5):CD003280. doi: 10.1002/14651858.CD003280.pub5. Cochrane Database Syst Rev. 2017. PMID: 28481421 Free PMC article.
References
-
- Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet392, 75–87. 10.1016/S0140-6736(18)30696-2 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM-149657/National Institute of General Medical Sciences,United States
- IK6BX006192/U.S. Department of Veterans Affairs
- RM1-GM-139690/National Institute of General Medical Sciences,United States
- IK6 BX006192/BX/BLRD VA/United States
- R01 GM-139046/GM/NIGMS NIH HHS/United States
- R35 GM126928/GM/NIGMS NIH HHS/United States
- R35 GM-134880/National Institute of General Medical Sciences,United States
- G102983-6263608307-1/U.S. Department of Defense
- R35 GM142481/GM/NIGMS NIH HHS/United States
- R35 GM-140881/National Institute of General Medical Sciences,United States
- R35 GM-126928/National Institute of General Medical Sciences,United States
- R01 GM139046/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

