Caerin 1.1 and 1.9 peptides induce acute caspase 3/GSDME-mediated pyroptosis in epithelial cancer cells
- PMID: 40251208
- PMCID: PMC12008296
- DOI: 10.1038/s41598-025-96438-0
Caerin 1.1 and 1.9 peptides induce acute caspase 3/GSDME-mediated pyroptosis in epithelial cancer cells
Abstract
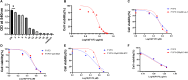
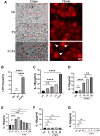
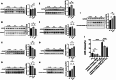
Caerin peptides exhibit a dual role in cancer treatment by directly killing cancer cells and modulating the tumour microenvironment to enhance anti-tumour immunity. This study investigates the mechanisms underlying caerin 1.1/1.9-induced acute cell death in epithelial cancer cells and explores their therapeutic potential. HeLa, A549, and Huh-7 cancer cell lines were treated with caerin 1.1/1.9 peptides. Morphological observations, flow cytometry, lactate dehydrogenase (LDH) release, and IL-18 secretion assays revealed the occurrence of pyroptosis following treatment. Specifically, a 1-h treatment with caerin 1.1/1.9 induced pyroptosis in HeLa, A549, and Huh-7 cells, characterised by cell swelling, membrane bubbling, and the release of IL-18 and LDH. Western blotting confirmed the upregulation of pyroptosis markers, including caspase-3, cleaved caspase-3, and GSDME-N fragments. These findings highlight the significant role of caerin peptides in inducing acute pyroptosis, a form of programmed cell death that enhances the immunogenicity of dying cancer cells, thus potentially improving the effectiveness of immunotherapies. This research underscores the therapeutic potential of caerin 1.1/1.9 peptides in cancer treatment, providing a foundation for developing new anti-cancer strategies that leverage both direct cytotoxic effects and immune modulation to achieve more effective and sustained anti-tumour responses.
Keywords: Caerin peptide; Caspase 3/GSDME signalling pathway; IL-18; Lactate dehydrogenase; Pyroptosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Caerin 1.1/1.9 interfere KHDRBS1-DDX5 regulatory axis to induce IL-18 mediated pyroptosis in a HeLa cell tumour model.Sci Rep. 2025 Jul 30;15(1):27866. doi: 10.1038/s41598-025-12450-4. Sci Rep. 2025. PMID: 40738927 Free PMC article.
-
Cisplatin induces acute liver injury by triggering caspase-3/GSDME-mediated cell pyroptosis.Hepatobiliary Pancreat Dis Int. 2025 Apr;24(2):177-187. doi: 10.1016/j.hbpd.2024.09.010. Epub 2024 Sep 30. Hepatobiliary Pancreat Dis Int. 2025. PMID: 39419722
-
Caspase 3/GSDME-Mediated Corneal Epithelial Pyroptosis Promotes Dry Eye Disease.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):24. doi: 10.1167/iovs.66.1.24. Invest Ophthalmol Vis Sci. 2025. PMID: 39792075 Free PMC article.
-
The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review.Int J Biol Macromol. 2023 Jul 1;242(Pt 2):124832. doi: 10.1016/j.ijbiomac.2023.124832. Epub 2023 May 15. Int J Biol Macromol. 2023. PMID: 37196719 Review.
-
The multifaceted roles of GSDME-mediated pyroptosis in cancer: therapeutic strategies and persisting obstacles.Cell Death Dis. 2023 Dec 16;14(12):836. doi: 10.1038/s41419-023-06382-y. Cell Death Dis. 2023. PMID: 38104141 Free PMC article. Review.
Cited by
-
Synergistic Antitumor Effects of Caerin Peptides and Dendritic Cell Vaccines in a 4T-1 Murine Breast Cancer Model.Vaccines (Basel). 2025 May 28;13(6):577. doi: 10.3390/vaccines13060577. Vaccines (Basel). 2025. PMID: 40573908 Free PMC article.
-
Caerin 1.1/1.9 interfere KHDRBS1-DDX5 regulatory axis to induce IL-18 mediated pyroptosis in a HeLa cell tumour model.Sci Rep. 2025 Jul 30;15(1):27866. doi: 10.1038/s41598-025-12450-4. Sci Rep. 2025. PMID: 40738927 Free PMC article.
References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263. 10.3322/caac.21834 (2024). - PubMed
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74, 12–49. 10.3322/caac.21820 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

