Oncolytic virotherapy provides a potent therapy option for squamous bladder cancer
- PMID: 40251219
- PMCID: PMC12008219
- DOI: 10.1038/s41598-025-96419-3
Oncolytic virotherapy provides a potent therapy option for squamous bladder cancer
Abstract
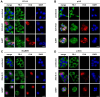
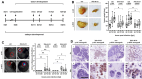
Prognosis for squamous cell carcinoma (SCC) of the bladder is limited mostly because of lack of effective treatment regimens. Oncolytic virotherapy represents a promising option for bladder cancer and received in 2024 FDA therapy designation for the treatment of non-invasive high-grade bladder cancer (BLCA). For muscle-invasive bladder cancer (MIBC), preclinical studies demonstrated high efficacy of the oncolytic adenovirus XVir-N-31 in urothelial carcinoma (UC). We analyzed the potency of XVir-N-31 virotherapy as a novel treatment option in SCC. Replication of XVir-N-31 has been described to be facilitated by high expression level of Y-Box binding protein 1 (YB-1). Increased YB-1-mRNA expression was detected in basal/squamous subtype in TCGA BLCA cohort compared to urothelial and luminal BLCA and correlated with patient outcomes. Furthermore, immunohistochemical staining of 89 SCC on a tissue microarray confirmed strong YB-1 expression in squamous BLCA (sq-BLCA). In vitro, XVir-N-31 showed in subtype-specific cell cultures high rates of infection, replication and cell-killing capacity. In a novel in ovo xenograft model, XVir-N-31 impaired growth of xenografts of patient-derived ex vivo cell lines (p-SCC, p-UC) with growth suppression rates of 39-49%. We provide preclinical evidence ex vivo and in ovo for high efficacy of XVir-N-31 based oncolytic virotherapy as novel SCC therapy.
Keywords: Bladder cancer; Oncolytic virus; Squamous cell carcinoma; Virotherapy; XVir-N-31; YB-1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

