Transcriptomic insights into the resistance mechanism of Penaeus vannamei against highly lethal Vibrio parahaemolyticus
- PMID: 40251246
- PMCID: PMC12008197
- DOI: 10.1038/s41598-025-96168-3
Transcriptomic insights into the resistance mechanism of Penaeus vannamei against highly lethal Vibrio parahaemolyticus
Abstract
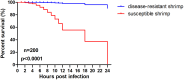
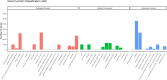
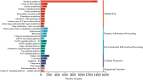
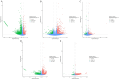
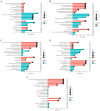
Highly lethal Vibrio disease (HLVD) caused by a virulent strain of Vibrio parahaemolyticus (VpHLVD), which poses a significant threat to Penaeus vannamei post-larvae, leads to substantial mortality and economic losses. To address this challenge, researchers have recently isolated a highly disease-resistant strain of P. vannamei shrimp. However, the underlying mechanisms that could improve disease resistance require further investigation. Our study found that disease-resistant shrimp exhibited a remarkable ability to prevent VpHLVD invasion effectively. To unravel the genetic basis of this resistance, we conducted a transcriptomic analysis with susceptible and disease-resistant shrimp at various time points (0, 6, and 12 h) post-infection with VpHLVD. Differential gene expression (DEGs) analysis of uninfected shrimp revealed that disease-resistant individuals displayed higher expression of immune-related genes and pathways compared to their susceptible counterparts. Simultaneously, they exhibited lower expression of Vibrio toxin-binding genes and Vibrio colonization gene, indicating enhanced defense mechanisms in the resistant shrimp. Upon VpHLVD infection, DEGs analysis also showed that susceptible shrimp attempt to mount a similar immune response as the disease-resistant shrimp during the early stages of infection. However, as the infection progresses, the defense strategies diverge between the two groups, with the peak of gene response occurring later in the disease-resistant shrimp. Our findings indicated that disease-resistant shrimp did not experience significant stress during the early stages of infection and are capable of effectively enhancing their immune response in the middle and late stages of the infection. In summary, our study enhanced the understanding of the mechanisms employed by disease-resistant shrimp to combat Vibrio, and would help to develop effective strategies for disease prevention and control, ultimately reducing the impact of HLVD on shrimp aquaculture.
Keywords: Penaeus vannamei; Vibrio parahaemolyticus; Highly lethal Vibrio disease; Transcriptomic analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

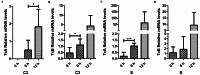

Similar articles
-
Differentially Expressed Genes in Hepatopancreas of Acute Hepatopancreatic Necrosis Disease Tolerant and Susceptible Shrimp (Penaeus vannamei).Front Immunol. 2021 May 13;12:634152. doi: 10.3389/fimmu.2021.634152. eCollection 2021. Front Immunol. 2021. PMID: 34054803 Free PMC article.
-
Transcriptome analysis of Pacific white shrimp (Litopenaeus vannamei) challenged by Vibrio parahaemolyticus reveals unique immune-related genes.Fish Shellfish Immunol. 2018 Jun;77:164-174. doi: 10.1016/j.fsi.2018.03.030. Epub 2018 Mar 19. Fish Shellfish Immunol. 2018. PMID: 29567139
-
Comparative transcriptome analysis of hepatopancreas reveals the potential mechanism of shrimp resistant to Vibrio parahaemolyticus infection.Fish Shellfish Immunol. 2024 Jan;144:109282. doi: 10.1016/j.fsi.2023.109282. Epub 2023 Dec 9. Fish Shellfish Immunol. 2024. PMID: 38081442
-
Unveiling the biofloc culture potential: Harnessing immune functions for resilience of shrimp and resistance against AHPND -causing Vibrio parahaemolyticus infection.Fish Shellfish Immunol. 2024 Aug;151:109710. doi: 10.1016/j.fsi.2024.109710. Epub 2024 Jun 18. Fish Shellfish Immunol. 2024. PMID: 38901683 Review.
-
Global landscape of Vibrio parahaemolyticus research: a bibliometric analysis.World J Microbiol Biotechnol. 2025 Jan 23;41(2):45. doi: 10.1007/s11274-025-04262-5. World J Microbiol Biotechnol. 2025. PMID: 39843643 Review.
Cited by
-
The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial.Int J Mol Sci. 2025 Jul 8;26(14):6557. doi: 10.3390/ijms26146557. Int J Mol Sci. 2025. PMID: 40724806 Free PMC article.
References
-
- Aguirre-Guzmán, G., Vázquez-Juárez, R. & Ascencio, F. Differences in the susceptibility of American white shrimp larval substages (Litopenaeusvannamei) to four vibrio species. J. Invertebr Pathol.78, 215–219. 10.1006/jipa.2001.5073 (2001). - PubMed
-
- Lavilla-Pitogo, C. R., Leano, E. M. & Paner, M. G. Mortalities of pond-cultured juvenile shrimp, Penaeusmonodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture164, 337–349 (1998).
-
- Lavilla-Pitogo, C. R., Baticados, M. C. L., Cruz-Lacierda, E. R. & de la Pena, L. D. Occurrence of luminous bacterial disease of Penaeusmonodon larvae in the Philippines. Aquaculture91, 1–13. 10.1016/0044-8486(90)90173-K (1990).
-
- de la Peña, L. D. et al. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeusvannamei and P.monodon cultured in the Philippines. Dis Aquat Organ116, 251–254. 10.3354/dao02919 (2015). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

