Maintenance therapy with Azacitidine for patients with myeloid malignancies after allogeneic hematopoietic stem cell transplantation
- PMID: 40251252
- PMCID: PMC12008360
- DOI: 10.1038/s41598-025-98059-z
Maintenance therapy with Azacitidine for patients with myeloid malignancies after allogeneic hematopoietic stem cell transplantation
Abstract
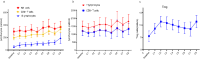
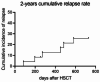
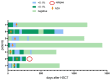
Disease relapse is a major cause of treatment failure after allogeneic haematopoietic stem cell transplantation (allo-HSCT) in patients with acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS). We retrospectively evaluated all patients (n = 145) with high-risk AML and MDS who underwent allo-HSCT between 2016 and 2022. Among them, 53 patients received maintenance therapy with azacitidine (AZA), and 10 of these patients received preemptive therapy. The rest of the 92 patients were in the control arm. The median follow-up time was 1215 days (103-3173 days). The median number of administered AZA cycles was 8. The 2-year relapse-free survival was 86.8% (46/53) in the AZA group compared with 76.1% (70/92) in the control group (P = 0.121). The 2-year overall survival in the AZA and control groups were 88.7% (47/53) and 82.6% (76/92), respectively (P = 0.326). Six patients (11.3%) in the AZA group experienced relapse, all of whom had a minimal residual disease (MRD)-positive status pre-HSCT. A total of 17 (32.1%) patients experienced grade 3-4 adverse events. In the preemptive therapy group, 70% (7/10) of the patients achieved complete remission after AZA treatment. AZA maintenance therapy following allo-HSCT is well tolerated and further follow-up and a larger group of patients will be necessary to confirm the potential to prevent relapse. The pre-transplant MRD status was identified as an important factor affecting the efficacy of AZA maintenance therapy.
Keywords: Acute myeloid leukemia; Allogeneic stem cell transplantation; Azacitidine; Minimal residual disease; Myelodysplastic syndromes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All procedures were performed in compliance with relevant laws. This study was approved by the ethical committee of the Institute of the Third Xiangya Hospital of Central South University, ethical approval number was 23409, date June 5, 2024. Consent for publication: The privacy rights of patients have been observed, Informed consent was obtained from available subjects involved in this retrospective study, and that the patient(s) (or relative/guardian) consented to the publishing of all clinical data, and other data included in the manuscript.
Figures


Similar articles
-
Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: results of the BMT-AZA prospective study.Ann Oncol. 2017 Jul 1;28(7):1547-1553. doi: 10.1093/annonc/mdx154. Ann Oncol. 2017. PMID: 28368509 Clinical Trial.
-
Azacitidine for Relapse After Allogeneic Stem Cell Transplantation-Single-Center Study.Transplant Proc. 2018 Sep;50(7):2212-2217. doi: 10.1016/j.transproceed.2018.02.148. Epub 2018 Mar 14. Transplant Proc. 2018. PMID: 30177138
-
Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial.Leukemia. 2012 Mar;26(3):381-9. doi: 10.1038/leu.2011.234. Epub 2011 Sep 2. Leukemia. 2012. PMID: 21886171 Free PMC article. Clinical Trial.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.Health Technol Assess. 2010 May;14 Suppl 1:69-74. doi: 10.3310/hta14Suppl1/10. Health Technol Assess. 2010. PMID: 20507806 Review.
-
Oral Azacitidine (CC-486) for the Treatment of Myeloid Malignancies.Clin Lymphoma Myeloma Leuk. 2022 Apr;22(4):236-250. doi: 10.1016/j.clml.2021.09.021. Epub 2021 Oct 8. Clin Lymphoma Myeloma Leuk. 2022. PMID: 34758945 Review.
References
-
- Schmid, C. et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood119, 1599–1606. 10.1182/blood-2011-08-375840 (2012). - PubMed
-
- Della Porta, M. G. et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood123, 2333–2342. 10.1182/blood-2013-12-542720 (2014). - PubMed
-
- Ciurea, S. O. et al. Relapse and survival after transplantation for complex karyotype acute myeloid leukemia: A report from the acute leukemia working party of the European society for blood and marrow transplantation and the university of Texas MD Anderson Cancer center. Cancer124, 2134–2141. 10.1002/cncr.31311 (2018). - PubMed
-
- Schmid, C. et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the chronic malignancies working party of the European society of blood and marrow transplantation. Haematologica103, 237–245. 10.3324/haematol.2017.168716 (2018). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

