Evaluation of early chemotherapy response by combining static- and dynamic [18F]FDG-PET with diffusion-weighted MRI in subcutaneous patient-derived endometrial cancer mouse models
- PMID: 40251389
- PMCID: PMC12008091
- DOI: 10.1186/s13550-025-01235-5
Evaluation of early chemotherapy response by combining static- and dynamic [18F]FDG-PET with diffusion-weighted MRI in subcutaneous patient-derived endometrial cancer mouse models
Abstract
Background: The combination of carboplatin and paclitaxel is the standard chemotherapy for treatment of high-risk and recurrent endometrial cancer. Evaluation of treatment response by diagnostic imaging is routinely carried out months after start of treatment, and is based on changes in tumor size or appearance of new metastases. The aim of this study was to evaluate early chemotherapeutic response in two subcutaneous endometrial cancer mouse models generated from patient-derived organoids using static- and dynamic [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) and diffusion-weighted (DW) magnetic resonance imaging (MRI). Mice were injected bilaterally with endometrioid endometrial cancer grade 3 (EEC G3), International Federation of Gynaecology and Obstetrics (FIGO) stage 3C1 (Model A) or stage 1B (Model B) organoids (n = 15 mice). The mice were randomized into treatment (combined carboplatin and paclitaxel, nA=8 / nB=6 tumors) or control (saline, nA=8 / nB=8 tumors) groups. During tumor progression, the mice underwent T2-weighted (T2w) MRI, DW-MRI and dynamic [18F]FDG-PET at baseline/Day 0 (start of treatment), Day 3 (early) and Day 10 (endpoint) using a sequential PET-MRI small-animal scanner.
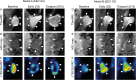
Results: At endpoint, tumor volumes at T2w-MRI (vMRI) were lower in the treatment groups in both models (p ≤ 0.029). The tumor metabolic rate (MRFDG) from dynamic PET, was significantly lower in the treatment group at the early timepoint (Day 3) and at the endpoint in Model A (p ≤ 0.042). In Model B, MRFDG was similar for both groups at Day 3 and at endpoint (p≥0.217). The 10 tumor voxels with the highest standardised uptake value (SUV10) from static [18F]-FDG-PET was significantly lower at endpoint in the treatment groups in both models (p ≤ 0.041), but not at the early timepoint (p≥0.083). Similarly, the tumor apparent diffusion coefficient (ADCmean) was significantly higher indicating treatment response at endpoint for treatment groups in both models (p ≤ 0.036).
Conclusions: Multimodal imaging is feasible for evaluation of early signs of treatment response in preclinical subcutaneous endometrial cancer models. The novel MRFDG dynamic PET imaging parameter seems most promising for detecting very early treatment response following chemotherapy.
Keywords: Chemotherapy; Diffusion-weighted MRI; Dynamic PET; Endometrial cancer; Metabolic rate of glucose; Organoid models; Preclinical imaging; Treatment response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The use of human tumor material was approved by the Regional Ethical Committee of Western Norway (REK Vest, Approval ID 2015/2333 and 2018/548). Animal experiments were approved by The Norwegian Food Safety Authority (Mattilsynet) in accordance with Norwegian and European regulations and guidelines (Approval ID 20194). Consent for publication: Not applicable. Competing interests: The authors declares that they have no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–49. - PubMed
-
- Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383(21):2053–64. - PubMed
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. - PubMed
-
- Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. International Journal of Gynecologic Cancer [Internet]. 2021 Jan 1 [cited 2023 Jan 5];31(1). Available from: https://ijgc.bmj.com/content/31/1/12 - PubMed
LinkOut - more resources
Full Text Sources
Medical

