Extension and validation of a physiologically based toxicokinetic model for risk assessment of aluminium exposure in humans
- PMID: 40251409
- PMCID: PMC12185619
- DOI: 10.1007/s00204-025-04031-1
Extension and validation of a physiologically based toxicokinetic model for risk assessment of aluminium exposure in humans
Abstract
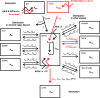
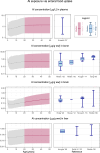
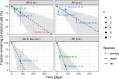
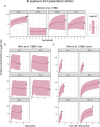
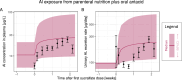
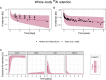
The safety of aluminium (Al) exposure from sources such as food, parenteral nutrition or adjuvanted medicinal products is still a matter of uncertainty. Since toxicokinetic studies in humans are lacking, model predictions are warranted for risk assessment. Recently, we established a physiologically based toxicokinetic (PBTK) model for Al built on a comprehensive toxicokinetic database, which could describe Al biokinetics in rats and human adults after single oral and intravenous doses of soluble Al salts. Since then, we have substantially amended the model, rendering it applicable to accurately represent children and their dynamically changing physiology (including maturating renal function in neonates and increased bone turnover during puberty). Also, additional sources of exposure were implemented, including vaccinations, subcutaneous allergen immunotherapies, food, antacids and parenteral nutrition. The model predictions in plasma and tissues were then compared to own published data and literature Al measurements after exposure from food (human reference values), parenteral nutrition (toxic levels in children and adults), adjuvanted allergen products or vaccines in rats and humans, and whole-body retention data. Al levels were predicted remarkably well, in plasma and toxicologically important tissues like bone, liver and brain. To our knowledge, this is the first Al PBTK model in humans ready for use in regulatory risk assessment, allowing to simulate Al exposure in children and adults from various sources of Al exposure like food and drinking water, Al contaminations in parenteral nutrition solutions, or poorly soluble Al complexes in medicinal products including Al-adjuvanted immunotherapeutics and vaccines.
Keywords: Aluminium; PBTK; Toxicokinetics; Validation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflict of interest or conflict of interest at the time of manuscript finalisation. All data, material and code are available in the electronic supplement.
Figures

Similar articles
-
Model-informed exploration of the boundaries of safe aluminium exposure from allergen immunotherapy in children.Pediatr Allergy Immunol. 2025 Aug;36(8):e70181. doi: 10.1111/pai.70181. Pediatr Allergy Immunol. 2025. PMID: 40856306 Free PMC article.
-
Estimation of Dermal Exposure to Volatile Organic Compounds (VOCs) from Feminine Hygiene Products: Integrating Measurement Data and Physiologically Based Toxicokinetic (PBTK) Model.Environ Health Perspect. 2025 Jun;133(6):67020. doi: 10.1289/EHP15418. Epub 2025 Jun 20. Environ Health Perspect. 2025. PMID: 40344599 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
-
- Advenier E, Landry C, Colomb V, Cognon C, Pradeau D, Florent M, Goulet O, Ricour C, Corriol O (2003) Aluminum Contamination of Parenteral Nutrition and Aluminum Loading in Children on Long-Term Parenteral Nutrition. Journal of Pediatric Gastroenterology and Nutrition 36(4):448–453, https://journals.lww.com/jpgn/fulltext/2003/04000/aluminum_contamination... - PubMed
-
- Alfrey AC (1978) Dialysis encephalopathy syndrome. Annu Rev Med 29(1):93–98. 10.1146/annurev.me.29.020178.000521 - PubMed
-
- Asín J, Molín J, Pérez M, Pinczowski P, Gimeno M, Navascués N, Muniesa A, de Blas I, Lacasta D, Fernández A, de Pablo L, Mold M, Exley C, de Andrés D, Reina R, Luján L (2018) Granulomas Following Subcutaneous Injection With Aluminum Adjuvant-Containing Products in Sheep. Vet Pathol 56(3):418–428. 10.1177/0300985818809142 - PubMed
-
- Becker LC, Boyer I, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA (2016) Safety Assessment of Alumina and Aluminum Hydroxide as Used in Cosmetics. Int J Toxicol 35(3):16S-33S. 10.1177/1091581816677948 - PubMed
-
- Bergfors E, Hermansson G, Nyström Kronander U, Falk L, Valter L, Trollfors B (2014) How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr 173(10):1297–1307. 10.1007/s00431-014-2318-2 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

