Phollow reveals in situ phage transmission dynamics in the zebrafish gut microbiome at single-virion resolution
- PMID: 40251446
- PMCID: PMC12055606
- DOI: 10.1038/s41564-025-01981-1
Phollow reveals in situ phage transmission dynamics in the zebrafish gut microbiome at single-virion resolution
Abstract
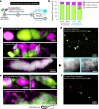
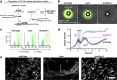
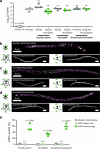
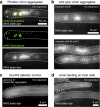
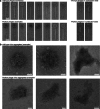
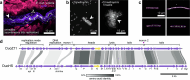
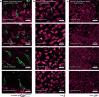
Bacteriophages show promise for microbiome engineering, but studying their transmission dynamics in multimember communities and animal hosts is technically challenging. We therefore created 'Phollow', a live imaging-based approach for tracking phage replication and spread in situ with single-virion resolution. Following interbacterial phage transmission is achieved by marking virions with distinct fluorescent proteins during assembly in newly infected cells. In vitro cell virology studies revealed clouds of phage virions dispersing upon bacterial lysis, leading to rampant transmission. Combining Phollow with optically transparent zebrafish, we visualized phage outbreaks within the vertebrate gut. We observed that virions from a zebrafish-derived Plesiomonas strain, but not a human-derived E. coli, rapidly disseminate systemically to the liver and brain. Moreover, antibiotics triggered waves of interbacterial transmission and sudden shifts in gut community ecology. Phollow ultimately empowers multiscale investigations of phage transmission and transkingdom interactions that have the potential to open new avenues for phage-based microbiome therapies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Phollow: Visualizing Gut Bacteriophage Transmission within Microbial Communities and Living Animals.bioRxiv [Preprint]. 2024 Jun 13:2024.06.12.598711. doi: 10.1101/2024.06.12.598711. bioRxiv. 2024. PMID: 38915633 Free PMC article. Preprint.
-
Abundance measurements reveal the balance between lysis and lysogeny in the human gut microbiome.Curr Biol. 2025 May 19;35(10):2282-2294.e11. doi: 10.1016/j.cub.2025.03.073. Epub 2025 Apr 28. Curr Biol. 2025. PMID: 40300605
-
The virulent bacteriophage Henu8 as an antimicrobial synergist against Escherichia coli.Microbiol Spectr. 2025 Jul;13(7):e0163324. doi: 10.1128/spectrum.01633-24. Epub 2025 May 16. Microbiol Spectr. 2025. PMID: 40377308 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
References
-
- Breitbart, M., Bonnain, C., Malki, K. & Sawaya, N. A. Phage puppet masters of the marine microbial realm. Nat. Microbiol.3, 754–766 (2018). - PubMed
-
- Chevallereau, A., Pons, B. J., van Houte, S. & Westra, E. R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol.20, 49–62 (2022). - PubMed
-
- Dion, M. B., Oechslin, F. & Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol.18, 125–138 (2020). - PubMed
-
- Correa, A. M. S. et al. Revisiting the rules of life for viruses of microorganisms. Nat. Rev. Microbiol.19, 501–513 (2021). - PubMed
MeSH terms
Grants and funding
- F32 AI112094/AI/NIAID NIH HHS/United States
- DP2AI154420/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P500PB_214357/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- DP2 AI154420/AI/NIAID NIH HHS/United States
- DP2 AI171121/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

