Effect of Saccharomyces boulardii supplementation to bismuth quadruple therapy on Helicobacter pylori eradication
- PMID: 40251486
- PMCID: PMC12008914
- DOI: 10.1186/s12876-025-03879-y
Effect of Saccharomyces boulardii supplementation to bismuth quadruple therapy on Helicobacter pylori eradication
Abstract
Background: Helicobacter pylori (H. pylori) infection is a common chronic infection, and there are over half of the global population infected with H. pylori. It is still controversial whether the supplementation of Saccharomyces boulardii (S. boulardii) to bismuth quadruple therapy is beneficial for H. pylori eradication.
Aim: To determine the effects of S. boulardii supplementation to bismuth quadruple therapy on H. pylori eradication.
Methods: We performed a systematic literature search across PubMed, Embase, Web of Science, and China National Knowledge Infrastructure for articles published up to October 2023. We calculated the pooled relative risk (RR) with the 95% confidence interval (CI). Statistical analyses were conducted using Stata/SE 15.1 software.
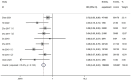
Results: Ten randomized controlled trials were included. Notably, S. boulardii supplementation to bismuth quadruple therapy significantly improved H. pylori eradication rates (RR = 1.08, 95% CI: 1.04-1.12) and reduced the incidence of total adverse effects (RR = 0.53, 95% CI: 0.45-0.62). Specifically, it reduced the incidence of some gastrointestinal adverse effects and nonspecific adverse effects, including diarrhea (RR = 0.28, 95% CI: 0.22-0.36), constipation (RR = 0.32, 95% CI: 0.18-0.55), abdominal distention (RR = 0.39, 95% CI: 0.26-0.59), nausea (RR = 0.59, 95% CI: 0.36-0.97), and rash (RR = 0.49, 95% CI: 0.28-0.86). In the subgroup analysis, long-term eradication duration (> 10 days; RR = 1.08, 95% CI: 1.04-1.13) and S. boulardii supplementation to be started and stopped at the same time as eradication treatment (RR = 1.09, 95% CI: 1.04-1.14) were found to significantly improve the eradication rate regardless of the S. boulardii dose (500 mg/day, RR = 1.10, 95% CI: 1.03-1.17; 1000 mg/day, RR = 1.08, 95% CI: 1.03-1.12).
Conclusions: The addition of S. boulardii to bismuth quadruple therapy significantly increased H. pylori eradication rates and decreased the adverse effects. We recommend adding 500 mg/day S. boulardii concurrently with bismuth quadruple therapy and continuing this therapy for > 10 days for optimal H. pylori eradication efficacy.
Keywords: Saccharomyces boulardii; Bismuth quadruple therapy; Helicobacter pylori; Meta-analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not Applicable. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests. PRISMA 2009 checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Figures


Similar articles
-
Efficacy and safety of Saccharomyces boulardii as an adjuvant therapy for the eradication of Helicobacter pylori: a meta-analysis.Front Cell Infect Microbiol. 2025 Feb 12;15:1441185. doi: 10.3389/fcimb.2025.1441185. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40012609 Free PMC article.
-
The effect of Saccharomyces boulardii supplementation on Helicobacter pylori eradication in children: a systematic review and meta-analysis of Randomized controlled trials.BMC Infect Dis. 2023 Dec 15;23(1):878. doi: 10.1186/s12879-023-08896-4. BMC Infect Dis. 2023. PMID: 38102568 Free PMC article.
-
[Influence of different timing of Saccharomyces boulardii combined with bismuth quadruple therapy for Helicobacter pylori eradication].Zhonghua Yi Xue Za Zhi. 2019 Jun 11;99(22):1731-1734. doi: 10.3760/cma.j.issn.0376-2491.2019.22.010. Zhonghua Yi Xue Za Zhi. 2019. PMID: 31216821 Clinical Trial. Chinese.
-
Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis.Helicobacter. 2019 Oct;24(5):e12651. doi: 10.1111/hel.12651. Epub 2019 Aug 14. Helicobacter. 2019. PMID: 31414551
-
Saccharomyces boulardii Allows Partial Patients to Avoid Reusing Bismuth Quadruple for Helicobacter pylori Rescue Therapy: A Single-Center Randomized Controlled Study.Front Cell Infect Microbiol. 2022 Jul 8;12:903002. doi: 10.3389/fcimb.2022.903002. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35880079 Free PMC article. Clinical Trial.
References
-
- Crowe SE. Helicobacter pylori infection. New Engl J Med. 2019;380:1158–65. [PMID: 30893536 10.1056/NEJMcp1710945]. - PubMed
-
- Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O’Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022. [PMID: 35944925 10.1136/gutjnl-2022-327745]. - PubMed
-
- Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The ideal Helicobacter pylori treatment for the present and the future. Digestion. 2022;103:62–8. [PMID: 34662879 10.1159/000519413]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

