Structure and function of vimentin in the generation and secretion of extracellular vimentin in response to inflammation
- PMID: 40251523
- PMCID: PMC12007377
- DOI: 10.1186/s12964-025-02194-z
Structure and function of vimentin in the generation and secretion of extracellular vimentin in response to inflammation
Abstract
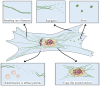
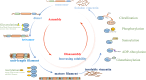
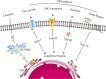
The canonical functions of vimentin in cell mechanics and migration have been recently expanded by the discovery of new roles for extracellular vimentin (ECV) in immune responses to infection, injury and cancer. In contrast with the predominantly filamentous form of intracellular vimentin, ECV exists largely as soluble oligomers. The release of ECV from intact cells is dependent on mechanisms that regulate the assembly and disassembly of intracellular vimentin, which are influenced by discrete post-translational modifications. In this review we highlight the processes that promote the conversion of intracellular and insoluble vimentin filaments to ECV and secretion mechanisms. Insights into the regulation of ECV release from stromal and immune cells could provide new diagnostic and therapeutic approaches for assessing and controlling inflammatory diseases.
Keywords: Extracellular vimentin (ECV); Inflammation; Post-translational modifications (PTMs); Unconventional protein secretion (UPS); Vimentin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Annexin A2 Contributes to Release of Extracellular Vimentin in Response to Inflammation.FASEB J. 2025 May 15;39(9):e70621. doi: 10.1096/fj.202500793R. FASEB J. 2025. PMID: 40346842 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Continuous self-repair protects vimentin intermediate filaments from fragmentation.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2417660122. doi: 10.1073/pnas.2417660122. Epub 2025 Jun 13. Proc Natl Acad Sci U S A. 2025. PMID: 40512788
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. - PubMed
-
- Parvanian S, Yan F, Su D, Coelho-Rato LS, Venu AP, Yang P, Zou X, Jiu Y, Chen H, Eriksson JE, Cheng F. Exosomal vimentin from adipocyte progenitors accelerates wound healing. Cytoskeleton (Hoboken). 2020;77:399–413. - PubMed
-
- Adolf A, Rohrbeck A, Munster-Wandowski A, Johansson M, Kuhn HG, Kopp MA, Brommer B, Schwab JM, Just I, Ahnert-Hilger G, Holtje M. Release of astroglial vimentin by extracellular vesicles: modulation of binding and internalization of C3 transferase in astrocytes and neurons. Glia. 2019;67:703–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

