Pre-referral injectable artesunate and outcomes of childhood severe malaria at a secondary health facility in North-central Nigeria: a cross-sectional study
- PMID: 40251537
- PMCID: PMC12008896
- DOI: 10.1186/s12936-025-05317-6
Pre-referral injectable artesunate and outcomes of childhood severe malaria at a secondary health facility in North-central Nigeria: a cross-sectional study
Abstract
Background: The use of pre-referral injectable artesunate is among the strategies adopted to improve the outcome of childhood severe malaria in Nigeria. However, the extent of its implementation and impact on outcomes remain unknown. This study assessed the pre-referral treatment with injectable artesunate for severe malaria, associated factors, and hospitalisation outcomes (discharge or death) among a cohort of children managed at a secondary health facility in Nigeria.
Methods: This cross-sectional study included children diagnosed with severe malaria admitted to a secondary health facility in Nigeria. Data on pre-referral treatment with injectable artesunate and other medications, demographics, clinical features, and outcomes among children were prospectively gathered and analysed them using SPSS.
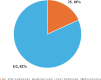
Results: Of the 137 children recruited, 7(6.3%) received pre-referral injectable artesunate; other medications received included antipyretics (53;47.3%), antibiotics (16;14.3%), artemisinin-based combination therapy (14;12.5%), and supplements (11;9.8%). Pre-referral injectable artesunate treatment was not associated with age, sex, and socioeconomic status (p > 0.05), and most of the clinical features except impaired consciousness with an adjusted odds ratio of 17.876 (95% CI 2.050 to 155.883). Of the 137 children, two deaths occurred, with a crude mortality rate of 1.5% (95% CI 0.04-5.2%). Pre-referral injectable artesunate treatment was not associated with hospitalisation durations and outcomes (death or discharge).
Conclusion: This study showed a very low uptake of pre-referral injectable artesunate among children with severe malaria, and the presence of impaired consciousness increased the odds of a child receiving injectable artesunate. In addition, most children with severe malaria had received pre-hospitalisation medications that were mostly inappropriate.
Keywords: Children; Outcomes; Pre-referral injectable artesunate; Severe malaria.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Kwara State Ministry of Health Ethics Review Committee. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the parents and guardians of all participating children. Data were maintained with strict confidentiality, secured in a password-protected computer, and de-identified during the analysis process. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- WHO. World malaria report. addressing inequity in the global malaria response. Geneva: World Health Organization; 2024. p. 2024.
-
- WHO Regional Office for Africa. Report on malaria in Nigeria 2022. Brazzaville: World Health Organization; 2023.
-
- WHO. World malaria report. Geneva: World Health Organization; 2023.
-
- Venkatesan P. WHO World malaria report La. ncet Microbe. 2023;2024(5): e214. - PubMed
-
- WHO. The use of rectal artesunate as a pre-referral treatment for severe Plasmodiumfalciparum malaria-2023 Update. Geneva: World Health Organization; 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical