Tyrosine kinase inhibitors in Ewing's sarcoma: a systematic review
- PMID: 40251562
- PMCID: PMC12008964
- DOI: 10.1186/s12885-025-14130-y
Tyrosine kinase inhibitors in Ewing's sarcoma: a systematic review
Abstract
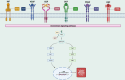
Ewing's sarcoma (ES) is a highly aggressive primary bone malignancy that primarily affects children and adolescents. Several tyrosine kinase receptors (RTKs) have been found to be overexpressed in ES samples, and it was demonstrated that some play significant roles in driving the malignant phenotype of ES. Specifically, ES with insulin-like growth factor 1 (IGF1R) or vascular endothelial growth factor (VEGFR) overexpression were correlated with more aggressive ES and worse outcomes. Other RTKs that were determined to be overexpressed in ES include platelet-derived growth factor receptor, stem cell factor receptor, and hepatocyte growth factor. Overexpression of these molecules suggests their possible tumor-driving role, making them potential targets for intervention. Various tyrosine kinase inhibitors (TKIs), including apatinib, anlotinib, and cabozantinib have shown clinical promise in patients with recurrent ES who have progressed on previous lines of therapy. The findings reported in this review emphasize the importance of assessing IGF1R-focused inhibitors and combinational therapeutic regimens in future research. Furthermore, biomarkers predictive of response are necessary to improve patient outcomes. In order to optimize ES care, considerations for patient eligibility on the basis of positivity for biomarkers predictive of response, and the inclusion of quality-of-life evaluations in studies must be addressed.
Keywords: Ewing’s Sarcoma; Receptors Tyrosine Kinase; Systematic Review; Tyrosine Kinase Inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not Applicable. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not Applicable.
Figures
References
-
- Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, et al. Ewing sarcoma. Nat Rev Dis Primers. 2018;4:5. - PubMed
-
- Esiashvili N, Goodman M, Marcus RB. Changes in incidence and survival of ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30:425–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical