HLA-F regulates the proliferation of trophoblast via PKM2-dependent glycolysis in the pathogenesis of preeclampsia
- PMID: 40251569
- PMCID: PMC12008859
- DOI: 10.1186/s10020-025-01201-w
HLA-F regulates the proliferation of trophoblast via PKM2-dependent glycolysis in the pathogenesis of preeclampsia
Abstract
Background: The regulatory molecule Human Leukocyte Antigen F (HLA-F) has been implicated in trophoblast proliferation during pregnancy, and reduced levels of this antigen have been identified in trophoblast cells of patients with preeclampsia. This study aimed to analyze the effect and mechanism of HLA-F on the proliferation of trophoblast and the underlying mechanism of reduced HLA-F involved in preeclampsia.
Methods: q-PCR, Western blot (WB), and Immunohistochemistry (IHC) were used to detect the expression of HLA-F and Pyruvate Kinase Muscle isoform 2 (PKM2) in placenta tissues. Jar cells were transfected with overexpression lentivirus, specific siRNA, and shRNA to regulate corresponding genes. Immunofluorescence was used to analyze the expression and distribution of HLA-F and PKM2. Extracellular and intracellular lactate, pyruvate, and enzymatic activity of PKM2 were measured using the corresponding assay kits. Cell proliferation was measured by CCK8, MTT, colony formation assay, and Mini patient-derived xenograft (Mini-PDX). Chromatin Immunoprecipitation and deep sequencing (ChIP-seq) and 4-dimensional label-free quantitative proteomics (4D-LFQP-LA) were used to analyze the HLA-F-binding DNA sequences and the differential lactylation proteins in HLA-F-overexpression Jar and its control.
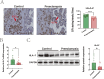
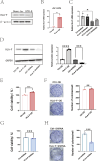
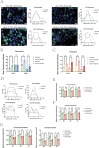
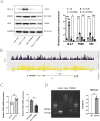
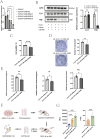
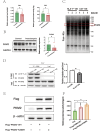
Results: The expression of HLA-F is reduced in extravillous trophoblast and villous cytotrophoblast from patients with preeclampsia. Over-expression of HLA-F promoted proliferation while under-expression inhibited it. Further experiments demonstrated that over-expression of HLA-F promoted expression of the PKM2 protein and its enzymatic activity, resulting in enhanced glycolysis in Jar cells. Specifically, we determined that HLA-F regulated the expression of PKM2 by binding the promoter of PKM, and promoted PKM2 enzyme activity by down-regulating the lactylation of residue K305. Moreover, silencing PKM2 with siRNA reduced HLA-F-mediated glycolysis and proliferation in HLA-F-overexpressing Jar cells. Finally, we corroborated these results using a MiniPDX model, with which we confirmed that the PKM2 agonist TEPP-46 promoted the proliferation of ShHLA-F Jar cells.
Conclusions: The reduced expression of HLA-F in placental trophoblast cells resulted in the downregulation of both PKM2 transcription and protein expression. Concurrently, the relative upregulation of lactylation at PKM2 K305 contributed to a decline in enzyme activity, further exacerbating glycolysis dysfunction. Collectively, these alterations led to a suppression of trophoblast proliferation capacity and involvement in the pathogenesis of preeclampsia.
Keywords: HLA-F; Lactylation; PKM2; Preeclampsia; Proliferation; Trophoblast.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and participation consent: The study protocol was approved by the Ethics Committee of Sichuan Provincial People’s Hospital (No. 2022 − 311). All participants signed the informed consent. Consent for publication: All authors provided consent for publication. Competing interests: The authors declare no competing interests.
Figures
References
-
- Anastasiou D, Yu Y, Israelsen WJ, Jiang J-K, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Brian P, Fiske KD, Courtney S, Malstrom TM, Khan C, Kung AP Skoumbourdis, Henrike Veith, Noel Southall, Walsh MJ, Brimacombe KR, Leister W, Sophia Y, Lunt, Zachary R, Johnson KE, Yen K, Kunii SM, Davidson HR, Christofk CP Austin, James Inglese, Marian, Harris H, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park H-W, Cantley LC, Thomas CJ. and Matthew G. Vander Heiden. ‘Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis’. Nature Chemical Biology. 2012;8:839– 47. - PMC - PubMed
-
- Bloxam DL, Bridget E, Bullen, Barry NJ, Walters, Lao TT. Placental Glycolysis and energy metabolism in preeclampsia. Am J Obstet Gynecol. 1987;157:97–101. - PubMed
-
- Buttura RV, Ramalho J, Lima ThálittaHA, Donadi EA, Luciana C, Veiga-Castelli CT, Mendes-Junior. and Erick C. Castelli. ‘HLA-F displays highly divergent and frequent haplotype lineages associated with different mRNA expression levels’. Hum Immunol. 2019;80:112–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

