Chemical reprogramming culture for the expansion of salivary gland epithelial basal progenitor cells
- PMID: 40251601
- PMCID: PMC12008940
- DOI: 10.1186/s13287-025-04295-5
Chemical reprogramming culture for the expansion of salivary gland epithelial basal progenitor cells
Abstract
Background: Salivary gland (SG) hypofunction presents a significant clinical challenge with limited treatment options. SG epithelial cells offer a promising approach due to their intrinsic tissue specificity and regenerative potential. However, the lack of efficient culture methods has hindered their clinical use.
Methods: This study presents a chemical reprogramming culture (CRC) system that utilizes a combination of three small molecules for the long-term two-dimensional culture of human SG epithelial progenitor cells. We characterized the cultured cells, measured their organoid-forming efficiencies, and assessed their differentiation potential. To evaluate the therapeutic efficacy of the SG basal progenitor cells (SG-BPCs), we administered them into a mouse model with radiation-induced SG hypofunction and assessed the functional recovery.
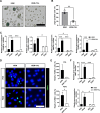
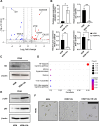
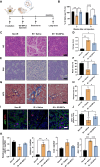
Results: By utilizing optimal concentrations of the small molecules Y-27632, A83-01, and LDN193189, the SG epithelial cells achieved over 50 population doubling levels (PD) within 80 d, surpassing the Hayflick limit. β-galactosidase and Terminal deoxynucleotidyl transferase dUTP nick end labeling staining confirmed that these small molecules inhibited cellular senescence and apoptosis, respectively. The cells expressed SG basal ductal cell markers KRT5, KRT19, and SOX9, with increased expression levels observed from PD5 to PD40. Notably, these expanded cells were able to differentiate into various SG cell types, including acinar and myoepithelial cells, indicating that SG-basal progenitor cells (SG-BPCs) were selectively proliferated using our CRC method. To assess the therapeutic potential of the expanded SG-BPCs, they were administered to mice with radiation-induced SG hypofunction. The treatment successfully restored SG function.
Conclusion: Our findings demonstrate that our CRC system is an effective method for the long-term culture of SG-BPCs. This advancement holds significant promise for the development of SG epithelial progenitor-based therapies to treat SG hypofunction.
Keywords: Basal progenitor cell; Cell therapy; Chemical reprogramming culture; Epithelial stem cell; Salivary gland.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Human SGs were provided by Yonsei University Severance Hospital, following the acquisition of informed consent and approval from the institutional review board. The human experiment protocol titled “A biobanking study to investigate the mechanisms underlying chemoresistance in head and neck cancer” was approved by the Yonsei University Severance Hospital on March 25, 2017 (permission number #4–2017-0325). All participants provided informed consent. The animal experiment protocol titled “Development of therapeutics for salivary gland hypofunction based on functional exosomes from human salivary gland stem cells through animal models” was approved by the Institutional Animal Care and Use Committee of Yonsei University College of Medicine on June 2, 2023 (approval number #2023–0192). The animal experiments have been reported in line with the ARRIVE guidelines 2.0. Consent for publication: The authors confirm their consent for publication. Competing interests: All authors declare no conflict of interests. Artificial intelligence: The authors declare that artificial intelligence-generated work was not used in this manuscript.
Figures



Similar articles
-
A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids.J Tissue Eng Regen Med. 2019 Mar;13(3):495-508. doi: 10.1002/term.2809. Epub 2019 Mar 6. J Tissue Eng Regen Med. 2019. PMID: 30666813
-
Three-Dimensional Culture of Salivary Gland Stem Cell in Orthotropic Decellularized Extracellular Matrix Hydrogels.Tissue Eng Part A. 2019 Oct;25(19-20):1396-1403. doi: 10.1089/ten.TEA.2018.0308. Epub 2019 Mar 18. Tissue Eng Part A. 2019. PMID: 30632466
-
Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals.Stem Cell Reports. 2016 Jan 12;6(1):150-62. doi: 10.1016/j.stemcr.2015.11.009. Epub 2015 Dec 24. Stem Cell Reports. 2016. PMID: 26724906 Free PMC article.
-
Unveiling Stem Cell Heterogeneity Toward the Development of Salivary Gland Regenerative Strategies.Adv Exp Med Biol. 2019;1123:151-164. doi: 10.1007/978-3-030-11096-3_9. Adv Exp Med Biol. 2019. PMID: 31016599 Review.
-
Immunocytochemistry of myoepithelial cells in the salivary glands.Prog Histochem Cytochem. 2003;38(4):343-426. doi: 10.1016/s0079-6336(03)80001-3. Prog Histochem Cytochem. 2003. PMID: 14509196 Review.
References
-
- Rehman HU. Sjögren’s syndrome. Yonsei Med J. 2003;44:947–54. - PubMed
-
- Takashi I, et al. Recent progress in regenerative therapy for damaged salivary: glands from bench to bedside. Oral Dis. 2024;30:38–49. 10.1111/odi.14692. - PubMed
-
- Abd El-Haleem MR, Selim AO, Attia GM. Bone marrow-derived mesenchymal stem cells ameliorate parotid injury in ovariectomized rats. Cytotherapy. 2018;20:204–17. 10.1016/j.jcyt.2017.10.003. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

