Phosphatidate phosphatase Lipin1 alters mitochondria-associated endoplasmic reticulum membranes (MAMs) homeostasis: effects which contribute to the development of diabetic encephalopathy
- PMID: 40251630
- PMCID: PMC12008933
- DOI: 10.1186/s12974-025-03441-3
Phosphatidate phosphatase Lipin1 alters mitochondria-associated endoplasmic reticulum membranes (MAMs) homeostasis: effects which contribute to the development of diabetic encephalopathy
Abstract
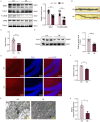
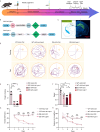
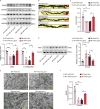
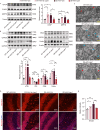
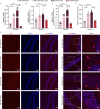
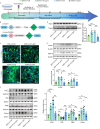
Diabetic encephalopathy (DE) is a common, chronic central nervous system complication of diabetes mellitus, and represents a condition without a clear pathogenesis or effective therapy. Findings from recent studies have indicated that a dyshomeostasis of mitochondria-associated endoplasmic reticulum membranes (MAMs) may be involved in the development of neurodegenerative diseases such as DE. MAMs represent a dynamic contact site between mitochondrial and endoplasmic reticulum (ER) membranes, where phospholipid components are exchanged with each other. Previous work within our laboratory has revealed that Lipin1, a critical enzyme related to phospholipid synthesis, is involved in the pathogenesis of DE. Here, we show that Lipin1 is downregulated within the hippocampus of a DE mouse model, an effect which was accompanied with a decrease in MAMs. Knockdown of Lipin1 in the hippocampus of normal mice resulted in a reduction of MAMs, ER stress, abnormal mitochondrial function, as well as impaired synaptic plasticity and cognitive function. These same phenomena were observed in the DE model, while an upregulation of Lipin1 within the hippocampus of DE mice improved these symptoms. Low levels of Lipin1 in DE mice were also associated with neuroinflammation, while an overexpression of Lipin1 significantly ameliorated the neuroinflammation observed in DE mice. In conclusion, Lipin1 ameliorates pathological changes associated with DE in a mouse model via prevention of dyshomeostasis in MAMs. Such findings suggest that Lipin1 may be serve as a new potential target for the treatment of DE.
Keywords: Cognitive dysfunction; Diabetic encephalopathy; Lipin1; MAMs; Mitochondria.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Scientific Research Ethics Committee of the Second Hospital of Shandong University, in accordance with Declaration of Helsinki and the Guide for Care and Use of Laboratory Animals. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Lipin1 ameliorates cognitive ability of diabetic encephalopathy via regulating Ca2+ transfer through mitochondria-associated endoplasmic reticulum membranes.Int Immunopharmacol. 2025 Mar 26;150:114266. doi: 10.1016/j.intimp.2025.114266. Epub 2025 Feb 16. Int Immunopharmacol. 2025. PMID: 39961213
-
Phosphatidate phosphatase Lipin1 involves in diabetic encephalopathy pathogenesis via regulating synaptic mitochondrial dynamics.Redox Biol. 2024 Feb;69:102996. doi: 10.1016/j.redox.2023.102996. Epub 2023 Dec 12. Redox Biol. 2024. PMID: 38103341 Free PMC article.
-
Inhibition of SGLT2 protects podocytes in diabetic kidney disease by rebalancing mitochondria-associated endoplasmic reticulum membranes.Cell Commun Signal. 2024 Nov 7;22(1):534. doi: 10.1186/s12964-024-01914-1. Cell Commun Signal. 2024. PMID: 39511548 Free PMC article.
-
Mitochondria-Endoplasmic Reticulum Contacts: The Promising Regulators in Diabetic Cardiomyopathy.Oxid Med Cell Longev. 2022 Apr 11;2022:2531458. doi: 10.1155/2022/2531458. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450404 Free PMC article. Review.
-
The effect of mitochondrial-associated endoplasmic reticulum membranes (MAMs) modulation: New insights into therapeutic targets for depression.Neurosci Biobehav Rev. 2025 May;172:106087. doi: 10.1016/j.neubiorev.2025.106087. Epub 2025 Mar 1. Neurosci Biobehav Rev. 2025. PMID: 40031998 Review.
References
-
- DeJong RN. The nervous system complications of diabetes mellitus, with special reference to cerebrovascular changes. J Nerv Ment Dis. 1950;111(3):181–206.
-
- Xu T, Liu J, Li X-R, Yu Y, Luo X, Zheng X, et al. The mTOR/NF-κB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Mol Neurobiol. 2021;58(8):3848–62. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

