Adjuvant dendritic cell-based immunotherapy in melanoma: insights into immune cell dynamics and clinical evidence from a phase II trial
- PMID: 40251644
- PMCID: PMC12007200
- DOI: 10.1186/s12967-025-06403-8
Adjuvant dendritic cell-based immunotherapy in melanoma: insights into immune cell dynamics and clinical evidence from a phase II trial
Abstract
Background: Dendritic cells (DCs) are the most efficient antigen-presenting cells and play a central role in the immune system, orchestrating immune response against tumors. We previously demonstrated that DC-based vaccination effectively induces anti-tumor immunity, yet at the same time showing a robust safety profile, making this treatment a potential candidate for effective adjuvant immunotherapy. To explore this possibility, we designed a randomized phase II trial (EudraCT no. 2014-005123-27) to provide a complementary autologous DC vaccination to patients (pts) with resected stage III/IV melanoma.
Methods: Overall, a total of 18 eligible pts were included in this study, 10 of whom received 6 monthly DC vaccination cycles combined with IL-2 administration (arm A), and 8 pts were enrolled in the follow-up observational cohort (arm B). A deep immune biomarkers profiling by multiplex immunoassay, human leukocyte antigens (HLA) typing, multiparametric flow cytometry and in situ tumor microenvironment analysis was performed for the entire pts cohort. The immunological response was assessed in vivo by DTH test and ex vivo against selected melanoma-associated antigens applying the IFN-γ ELISPOT assay.
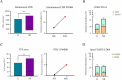
Results: Pts receiving DC vaccination showed a better relapse-free survival compared to the observational cohort (median 6.6 months, 95% CI, 2.3-not reached (nr) (arm A) vs 5.2 months, 95% CI, 2.5-nr (arm B), not significant), with a favorable trends for female pts (median 15.5 months, 95% CI, 2.6-nr (female) vs 3.3, 95% CI, 2.3-nr (male)), pts with less than 60 years (median 22.5 months, 95% CI, 2.6-nr (age < 60) vs 4.7 months, 95% CI, 2.3-nr (age ≥ 60), and pts with wild-type BRAF status (median 22.5 months, 95% CI, 8.6-nr (BRAF wt) vs 3.8 months, 95% CI, 2.3-nr (BRAF mutated). The toxicity profile was favourable, with no severe adverse events and only mild, manageable reactions. Moreover, additional immune response data suggested increased immune modulation in vaccinated patients, which may reflect a shift in immune dynamics.
Conclusions: Our findings support the safety and tolerability of DC vaccination as an adjuvant treatment for melanoma, demonstrating significant immune modulation at both the tumor site and peripherally in relapsed and non-relapsed patients. These results highlight the potential of autologous, personalised DC-based therapies and pave the way for the development of innovative immunotherapy combinations in future treatment strategies. Trial registration ClinicalTrials.gov NCT02718391; EudraCT no. 2014-005123-27.
Keywords: Dendritic cell; Immune modulatory; Immunotherapy; Skin cancer; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol was approved by the institutional Medical Ethical Review Board CEIIAV Ethics Committee (approval n° 1231 of 30/07/2015) and the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and later versions. Consent for publication: All participants involved in the study have provided written informed consent for the publication of their data and images in this manuscript. All authors have reviewed the manuscript and provided their consent for its publication. Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102(7):493–501. - PubMed
-
- Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30. - PubMed
-
- Long GV, Hauschild A, Santinami M, et al. Final results for adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2024;391(18):1709–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

