Malignant mesothelioma-associated inflammatory microenvironment promotes tumor progression via GPNMB
- PMID: 40251684
- PMCID: PMC12007160
- DOI: 10.1186/s12967-025-06407-4
Malignant mesothelioma-associated inflammatory microenvironment promotes tumor progression via GPNMB
Abstract
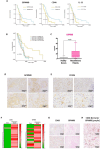
Background: Tumor-Associated Macrophages (TAMs) are the main immune component of the tumor stroma with heterogeneous functional activities, predominantly suppressing the immune response and promoting tumor progression, also via secretion of different factors. Among these, GPNMB (Glycoprotein non-metastatic B) is usually associated with disease progression in several tumor types. Malignant pleural mesothelioma (MPM) a severe neoplasia with poor prognosis, is characterized by an abundancy of TAMs, testifying the presence of a long-lasting inflammation which is pathogenetic of the disease. However, the role of GPNMB in MPM is unclear.
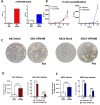
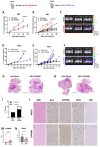
Methods: Clinical samples from patients with MPM were used to measure RNA and protein levels of GPNMB. The functional role of GPNMB in vivo was studied in an orthotopic mouse model of mesothelioma using the murine cell lines AB1 and AB22. Experiments included in vivo tumor growth in wild type and in GPNMB-deficient mice and blocking of GPNMB-induced signaling with anti-CD44 antibodies.
Results: We show that in human and murine MPM tissues the protein GPNMB is mainly produced by infiltrating TAMs. Gpnmb RNA levels in MPM patients from TCGA are significantly associated with lower survival. Using an orthotopic mouse model of mesothelioma we observed that in GPNMB-defective mice (DBA2/J mice) unable to produce the protein, tumors formed by AB1 and AB22 mesothelioma cells grow significantly less than in GPNMB-proficient mice (DBA2/J-Gpnmb+ mice), indicating that host GPNMB is involved in tumor progression. Likewise, the ectopic expression of GPNMB in AB1 and AB22 cells causes an acceleration of tumor growth in vivo, significantly different compared to mock-transduced cells. Treatment of tumor-bearing mice with blocking anti-CD44 (a major receptor for GPNMB) results in a significant reduction of tumor growth.
Conclusions: Overall, these results indicate that the protein GPNMB, a product and marker gene of TAMs, is a driver of mesothelioma progression and may constitute a promising therapeutic target.
Keywords: Anti-CD44; GPNMB; Malignant mesothelioma; Orthotopic model; Tumor-associated macrophages.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The methods were performed following the approved guidelines, and all experimental protocols were approved by the ethics committee of the IRCCS Humanitas Research Hospital, Rozzano (Milano), Italy. Consent for publication: All authors contributed to the article and approved the submitted version. Competing interests: No potential competing interests was associated with this manuscript.
Figures


Similar articles
-
Important functional role of the protein osteopontin in the progression of malignant pleural mesothelioma.Front Immunol. 2023 Jun 16;14:1116430. doi: 10.3389/fimmu.2023.1116430. eCollection 2023. Front Immunol. 2023. PMID: 37398648 Free PMC article.
-
Podoplanin promotes progression of malignant pleural mesothelioma by regulating motility and focus formation.Cancer Sci. 2017 Apr;108(4):696-703. doi: 10.1111/cas.13190. Epub 2017 Apr 12. Cancer Sci. 2017. PMID: 28182302 Free PMC article.
-
GAS5 long non-coding RNA in malignant pleural mesothelioma.Mol Cancer. 2014 May 23;13:119. doi: 10.1186/1476-4598-13-119. Mol Cancer. 2014. PMID: 24885398 Free PMC article.
-
Heterogeneous Contributing Factors in MPM Disease Development and Progression: Biological Advances and Clinical Implications.Int J Mol Sci. 2018 Jan 13;19(1):238. doi: 10.3390/ijms19010238. Int J Mol Sci. 2018. PMID: 29342862 Free PMC article. Review.
-
Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma.Int J Mol Sci. 2018 Mar 30;19(4):1041. doi: 10.3390/ijms19041041. Int J Mol Sci. 2018. PMID: 29601534 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

