Temporal Galactose-Manganese Feeding in Fed-Batch and Perfusion Bioreactors Modulates UDP-Galactose Pools for Enhanced mAb Glycosylation Homogeneity
- PMID: 40251805
- PMCID: PMC12152465
- DOI: 10.1002/bit.28999
Temporal Galactose-Manganese Feeding in Fed-Batch and Perfusion Bioreactors Modulates UDP-Galactose Pools for Enhanced mAb Glycosylation Homogeneity
Abstract
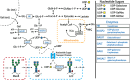
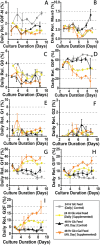
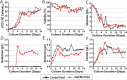
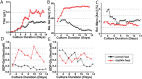
Monoclonal antibodies (mAbs) represent a majority of biotherapeutics in the market today. These glycoproteins undergo posttranslational modifications, such as N-linked glycosylation, that influence the structural & functional characteristics of the antibody. Glycosylation is a heterogenous posttranslational modification that may influence therapeutic glycoprotein stability and clinical efficacy, which is why it is often considered a critical quality attribute (CQA) of the mAb product. While much is known about the glycosylation pathways of Chinese Hamster Ovary (CHO) cells and how cell culture chemical modifiers may influence the N-glycosylation profile of the final product, this knowledge is often based on the final cumulative glycan profile at the end of the batch process. Building a temporal understanding of N-glycosylation and how mAb glycoform composition responds to real-time changes in the biomanufacturing process will help build integrated process models that may allow for glycosylation control to produce a more homogenous product. Here, we look at the effect of specific nutrient feed media additives (e.g., galactose, manganese) and feeding times on the N-glycosylation pathway to modulate N-glycosylation of a Herceptin biosimilar mAb (i.e., Trastuzumab). We deploy the N-GLYcanyzer process analytical technology (PAT) to monitor glycoforms in near real-time for bench-scale bioprocesses operated in both fed-batch and perfusion modes to build an understanding of how temporal changes in mAb N-glycosylation are dependent on specific media additives. We find that Trastuzumab terminal galactosylation is sensitive to media feeding times and intracellular nucleotide sugar pools. Temporal analysis reveals an increased desirable production of single and double galactose-occupied glycoforms over time under glucose-starved fed-batch cultures. Comparable galactosylation profiles were also observed between fed-batch (nutrient-limited) and perfusion (non-nutrient-limited) bioprocess conditions. In summary, our results demonstrate the utility of real-time monitoring of mAb glycoforms and feeding critical cell culture nutrients under fed-batch and perfusion bioprocessing conditions to produce higher-quality biologics.
Keywords: N‐linked glycosylation; bioprocessing; monoclonal antibody; perfusion.
© 2025 The Author(s). Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no competing financial interests. This article reflects the author's views and should not be construed to represent FDA's views or policies. Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the FDA.
Figures



References
-
- Braasch, K. (2015). Dielectric and Precursor Analysis to Study Metabolic Effects on CHO Cell Viability and Antibody Glycosylation (Vol. 13).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

