The Aquaporin-4 Expression and Localization in the Olfactory Epithelium Modulate the Odorant-Evoked Responses and Olfactory-Driven Behavior
- PMID: 40251831
- PMCID: PMC12185977
- DOI: 10.1002/glia.70024
The Aquaporin-4 Expression and Localization in the Olfactory Epithelium Modulate the Odorant-Evoked Responses and Olfactory-Driven Behavior
Abstract
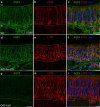
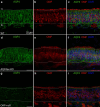
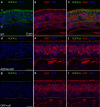
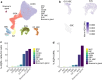
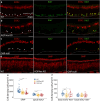
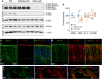
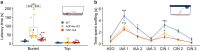
Aquaporin-4 (AQP4) is a water-selective channel expressed in glial cells throughout the central nervous system (CNS). It serves as the primary water channel in the neuropil and plays roles in physiological functions, including regulating water homeostasis by adjusting cell volume and modulating neuronal activity. Different isoforms of AQP4 are expressed in glial-like cells known as sustentacular cells (SUSs) of the olfactory epithelium (OE). Notably, mice lacking all AQP4 isoforms exhibit impaired olfactory abilities. Therefore, we aim to uncover the physiological role of AQP4 isoforms, particularly the AQP4ex isoforms (AQP4M1ex, AQP4M23ex) and the orthogonal array of particles (OAPs)-forming isoform (AQP4M23) in the OE. We investigated the impact of AQP4 isoforms on the OE, observing a reduced number of mature olfactory sensory neurons (OSNs), SUSs, and globose basal cells (GBCs) in mice lacking AQP4ex (AQP4ex-KO) or OAPs (OAP-null). This suggests that AQP4 isoforms are involved in maintaining an optimal microenvironment in the OE, preserving cell density. Next, we explored the role of AQP4 in modulating odorant-evoked responses through electro-olfactogram recordings, where we found reduced odorant responses in mice lacking AQP4 isoforms. Assessments of olfactory ability revealed deficits in odor-guided food-seeking behavior in both AQP4ex-KO and OAP-null mice. Furthermore, AQP4ex-KO mice exhibited a diminished ability to discriminate between different odorants, while OAP-null mice were unable to recognize them as distinct. Overall, our data highlight the role of AQP4 isoforms in modulating neuronal homeostasis, influencing odorant-evoked responses and cell density in the OE, with AQP4ex emerging as a key regulator despite its low abundance.
Keywords: AQP4 isoforms; AQP4M23; AQP4ex; Aquaporin‐4; olfactory epithelium; sustentacular cells.
© 2025 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Crucial role of Aquaporin-4 extended isoform in brain water Homeostasis and Amyloid-β clearance: implications for Edema and neurodegenerative diseases.Acta Neuropathol Commun. 2024 Oct 10;12(1):159. doi: 10.1186/s40478-024-01870-4. Acta Neuropathol Commun. 2024. PMID: 39385254 Free PMC article.
-
In mice, discrete odors can selectively promote the neurogenesis of sensory neuron subtypes that they stimulate.Elife. 2025 Jun 18;13:RP96152. doi: 10.7554/eLife.96152. Elife. 2025. PMID: 40531183 Free PMC article.
-
Deficiency of Aging-Related Gene Chitinase-Like 4 Impairs Olfactory Epithelium Homeostasis.Cell Prolif. 2025 Aug;58(8):e70055. doi: 10.1111/cpr.70055. Epub 2025 May 19. Cell Prolif. 2025. PMID: 40389328 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Afhami Mina, M. , Hashemi K., Afshari J., et al. 2018. “Acute Transplantation of Human Olfactory Mucosa‐Derived Olfactory Ensheathing Cells Fails to Improve Locomotor Recovery in Rats.” Acta Medica Iranica 56, no. 7.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

