Daily supplementation with lemon verbena extract decreases subjective energy and parental reports of hyperactivity in children displaying sub-clinical attention deficit hyperactivity disorder-type behaviours: A randomised controlled trial
- PMID: 40251851
- PMCID: PMC12287557
- DOI: 10.1177/02698811251324574
Daily supplementation with lemon verbena extract decreases subjective energy and parental reports of hyperactivity in children displaying sub-clinical attention deficit hyperactivity disorder-type behaviours: A randomised controlled trial
Abstract
Background: Current treatment options for attention deficit hyperactivity disorder (ADHD) are limited by factors such as adherence and cost, whilst no treatment options are available for sub-clinical or undiagnosed ADHD. Herbal preparations may therefore offer an alternative approach to the management of symptoms; Aloysia citriodora Paláu (lemon verbena) is a promising candidate.
Aim: To assess the behavioural, cognitive, psychological and physiological effects of 56 days of supplementation with lemon verbena extract (LVE) in children exhibiting symptoms of ADHD at the sub-clinical level.
Methods: This exploratory study followed a randomised, double-blind parallel groups design wherein 120 healthy participants aged 8-17 years received 15 mg/kg bw/d LVE or matched placebo for 56 days. Behavioural, cognitive, mood and physiological measures were collected in the lab at baseline and 28 and 56 days post-dose. Parents also evaluated the child's behaviour throughout the study.
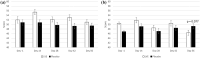
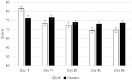
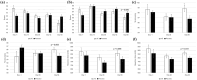
Results: Participants who received LVE reported greater subjective fatigue, defined as reduced energy levels according to the Profile of Mood States subscale, without impairments in cognitive performance across the 56-day intervention and lower depression symptoms on day 56, compared to placebo. The effect of LVE on parent ratings of hyperactive/impulsive behaviour also approached significance with fewer concerns being reported following the active treatment. Exploratory analyses showed further benefits to cognition and mood.
Conclusions: This study revealed novel, beneficial effects of LVE supplementation in children exhibiting a high frequency of behaviours characteristic of ADHD. Overall, LVE was safe and well-tolerated by participants, with no unexpected safety events.
Keywords: ADHD; Aloysia citriodora; Lemon verbena; Lippia citriodora; cognition.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CGS and AB are employed by Finzelberg GmbH & Co. KG, a company specialising in the production and distribution of botanical extracts. Their contribution to this publication pertains to specific aspects such as study design and proofreading of the manuscript but does not include data acquisition, processing or analysis. IP serves as a consultant to Finzelberg. The team focuses on research and development of new products.
Figures


Similar articles
-
Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years.Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD003018. doi: 10.1002/14651858.CD003018.pub3. Cochrane Database Syst Rev. 2011. PMID: 22161373 Free PMC article.
-
Methylphenidate for children and adolescents with autism spectrum disorder.Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011144. doi: 10.1002/14651858.CD011144.pub2. Cochrane Database Syst Rev. 2017. PMID: 29159857 Free PMC article.
-
Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD).Cochrane Database Syst Rev. 2023 Mar 27;3(3):CD009885. doi: 10.1002/14651858.CD009885.pub3. Cochrane Database Syst Rev. 2023. PMID: 36971690 Free PMC article.
-
Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD).Cochrane Database Syst Rev. 2015 Nov 25;2015(11):CD009885. doi: 10.1002/14651858.CD009885.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Mar 27;3:CD009885. doi: 10.1002/14651858.CD009885.pub3. PMID: 26599576 Free PMC article. Updated.
-
Folic acid with or without vitamin B12 for cognition and dementia.Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2. PMID: 14584018 Updated.
References
-
- Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. (2019) Aloysia citriodora Palau (lemon verbena) for insomnia patients: A randomised, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res 33: 350–359. - PubMed
-
- Bahramsoltani R, Rostamiasrabadi P, Shahpiri Z, et al. (2018) Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J Ethnopharmacol 222: 34–51. - PubMed
-
- Bano A, Hepsomali P, Rabbani F, et al. (2023) The possible “calming effect” of subchronic supplementation of a standardised phospholipid carrier-based Melissa officinalis L. extract in healthy adults with emotional distress and poor sleep conditions: Results from a prospective, randomised, double-blinded, placebo-controlled clinical trial. Front Pharmacol 14: 1250560. - PMC - PubMed
-
- Conners CK, Pitkanen J, Rzepa SR. (2011) Conners 3rd Edition (Conners 3; Conners 2008). In: Kreutzer JS, DeLuca J, Caplan B. (eds.) Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York, pp. 675–678.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

