Effect of host-protein test (TRAIL/IP-10/CRP) on antibiotic prescription and emergency department or urgent care center return visits: The JUNO pilot randomized controlled trial
- PMID: 40251855
- PMCID: PMC12435126
- DOI: 10.1111/acem.70031
Effect of host-protein test (TRAIL/IP-10/CRP) on antibiotic prescription and emergency department or urgent care center return visits: The JUNO pilot randomized controlled trial
Abstract
Objectives: Determining etiology for adults with symptoms of lower respiratory tract infection (LRTI) is challenging. MeMed BV (MMBV), an FDA-cleared blood test, computationally integrates the levels of three host proteins to differentiate bacterial and viral infections. We evaluated MMBV's impact on safe antibiotic prescribing at emergency department/urgent care centers (ED/UC).
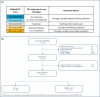
Methods: The JUNO randomized controlled trial (RCT; NCT05762302) was a prespecified pilot phase of the JUPITER RCT. JUNO enrolled adult ED/UC patients with LRTI symptoms and clinician's consideration for antibiotic treatment. Inclusion criteria were fever within 7 days and one of cough, sputum production, dyspnea, or auscultation abnormality. Exclusion criteria were prior antibiotic use or immunosuppression. Patients were randomized to standard care (SC) or SC plus MMBV arms. JUNO's primary objective was to assess antibiotic prescription rate in the SC arm; the secondary objective was to assess JUPITER's study design.
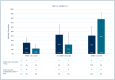
Results: Eleven centers randomized 260 patients, with 214 included (106 SC, 108 MMBV). Median (IQR) age was 40 (28-55.8) years, 57% were female, and 78.5% were enrolled at ED. Common symptoms were cough (93.0%) and chills (70.0%). Overall, antibiotic prescription rates were 30% (95% CI 22% to 40%) and 24% (95% CI 17% to 33%) in the SC arm versus the MMBV (absolute difference of -6% [95% CI -18% to 6%]). More antibiotics were given with bacterial MMBV scores (41% vs. 78%, absolute difference 37%, 95% CI 6% to 61%; n = 40) and less with viral MMBV scores (25% vs. 12%, absolute difference -13%, 95% CI -25% to 0%; n = 144) in the SC versus MMBV arms. There was no increase in ED/UC return visits (8% vs. 3%, difference -6%, 95% CI -12% to 1%) or hospitalizations (3% vs. 0%, difference -3%, 95% CI -7% to 1%) in the SC arm versus the MMBV arm.
Conclusions: JUNO demonstrated that JUPITER's design results in 30% antibiotic prescription rate in the SC arm. JUNO supports that MMBV optimizes antibiotic prescriptions without increasing return ED/UC visits or hospitalizations.
Keywords: antibiotics; bacterial; diagnostics; rapid host response test; viral.
© 2025 The Author(s). Academic Emergency Medicine published by Wiley Periodicals LLC on behalf of Society for Academic Emergency Medicine.
Conflict of interest statement
AJS—research grants from AstraZeneca, Brainbox, Spectral; consultant for AstraZeneca. CMC—research grants from Abbott, AstraZeneca, Brainbox, Roche, and MeMed. WFP—research grants from Abbott, Brainbox, CSL‐Vifor, Quidel, Roche, and Siemens; consultant for Abbott, Astra‐Zeneca, Biocogniv, Brainbox, Bristol Meyers Squibb, Janssen, Osler, Quidel, Roche, Siemens, Spinchip, and Werfen; stock/ownership interests in AseptiScope Inc., Brainbox Inc., Biocogniv, Inc., Braincheck Inc., Coagulo Inc., Comprehensive Research Associates LLC, Comprehensive Research Management Inc., Emergencies in Medicine LLC, Lucia Inc., Prevencio Inc., RCE Technologies, ROMTech, ScPharma, Trivirum Inc., and Upstream Inc. ACM—research grants from Abbott, AstraZeneca, Biomerieux, CDC, Hologic, MeMed, Vapotherm, and 1 Eq Inc.; consultant for Vapotherm and Biomerieux. TBN—received honoraria from Gilead, GSK, AstraZeneca, MSD, and Medison. The other authors declare no conflicts of interest.
Figures
References
-
- Houck PM, Bratzler DW. Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes? Curr Opin Infect Dis. 2005;18(2):151‐156. - PubMed
-
- Berjohn CM, Fishman NO, Joffe MM, Edelstein PH, Metlay JP. Treatment and outcomes for patients with Bacteremic pneumococcal pneumonia. Medicine. 2008;87(3):160‐166. - PubMed
-
- Schuetz P, Christ‐Crain M, Thomann R, et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059‐1066. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

