Effectiveness of Autologous Hematopoietic Stem Cell Transplantation versus Alemtuzumab and Ocrelizumab in Relapsing Multiple Sclerosis: A Single Center Cohort Study
- PMID: 40251896
- PMCID: PMC12278282
- DOI: 10.1002/ana.27247
Effectiveness of Autologous Hematopoietic Stem Cell Transplantation versus Alemtuzumab and Ocrelizumab in Relapsing Multiple Sclerosis: A Single Center Cohort Study
Abstract
Objective: To compare clinical and radiological outcomes among relapsing multiple sclerosis patients treated with autologous hematopoietic stem cell transplantation (AHSCT), alemtuzumab (ATZ), and ocrelizumab (OCR).
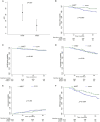
Methods: From a London (UK) hospital-based observational cohort, modeled data were obtained from 621 relapsing-remitting multiple sclerosis patients, who were treated with AHSCT (n = 103), ATZ (n = 204), and OCR (n = 314), and were followed up for 43, 43, and 32 median months, respectively. The annualized relapse rate, new magnetic resonance imaging (MRI) lesions, and disability progression on Expanded Disability Status Scale were assessed.
Results: AHSCT showed superior efficacy compared with ATZ and OCR. After 5-year follow up, the mean annualized relapse rate (0.026 vs 0.087; p < 0.001), the risk of relapses (HR 0.29, 95% CI 0.13-0.63; p = 0.002), and of MRI activity (HR 0.33, 95% CI 0.15-0.72; p = 0.006) were significantly lower in AHSCT versus ATZ group. Compared with OCR, after 3-year follow-up AHSCT showed a significantly lower annualized relapse rate (0.028 vs 0.073; p = 0.02) and a trend to reduced risk of relapse (HR 0.45, 95% CI 0.18-1.14; p = 0.09), but similar low rates (6%) of new MRI activity (HR 0.86, 95% CI 0.28-2.67; p = 0.80). In addition, there was a similar risk of Expanded Disability Status Scale progression in AHSCT, and both ATZ (HR 1.19, 95% CI 0.71-2.00; p = 0.50) and OCR (HR 1.08, 95% CI 0.57-2.04; p = 0.82) groups.
Interpretation: AHSCT was followed by greater prevention of relapses compared with ATZ and OCR, and suppressed more profoundly MRI activity than ATZ, but similarly to OCR, albeit with shorter follow up. The risk of accumulating disability was similar among the treated groups. Studies with larger sample sizes and longer follow up may enable confirmation of these findings or detection of any additional differential effects. ANN NEUROL 2025;98:294-307.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures


References
-
- Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune‐mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 2020;55:283–306. 10.1038/s41409-019-0684-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

