Lipid polymorphism of plant thylakoid membranes. The dynamic exchange model - facts and hypotheses
- PMID: 40251902
- PMCID: PMC12008737
- DOI: 10.1111/ppl.70230
Lipid polymorphism of plant thylakoid membranes. The dynamic exchange model - facts and hypotheses
Abstract
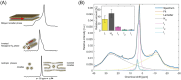
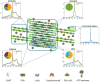
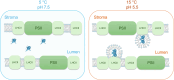
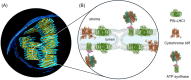
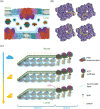
The light reactions of oxygenic photosynthesis are performed by protein complexes embedded in the lipid bilayer of thylakoid membranes (TMs). Bilayers provide optimal conditions for the build-up of the proton motive force (pmf) and ATP synthesis. However, functional plant TMs, besides the bilayer, contain an inverted hexagonal (HII) phase and isotropic phases, a lipid polymorphism due to their major, non-bilayer lipid species, monogalactosyldiacylglycerol (MGDG). The lipid phase behavior of TMs is explained within the framework of the Dynamic Exchange Model (DEM), an extension of the fluid-mosaic model. DEM portrays the bilayer phase as inclusions between photosynthetic supercomplexes - characterized by compromised membrane impermeability and restricted sizes inflicted by the segregation propensity of lipid molecules, safe-guarding the high protein density of TMs. Isotropic phases mediate membrane fusions and are associated with the lumenal lipocalin-like enzyme, violaxanthin de-epoxidase. Stromal-side proteins surrounded by lipids give rise to the HII phase. These features instigate experimentally testable hypotheses: (i) non-bilayer phases mediate functional sub-compartmentalization of plant chloroplasts - a quasi-autonomous energization and ATP synthesis of each granum-stroma TM assembly; and (ii) the generation and utilization of pmf depend on hydrated protein networks and proton-conducting pathways along membrane surfaces - rather than on strict impermeability of the bilayer.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Figures

Similar articles
-
Molecular level insight into non-bilayer structure formation in thylakoid membranes: a molecular dynamics study.Photosynth Res. 2025 Jun 11;163(3):36. doi: 10.1007/s11120-025-01156-3. Photosynth Res. 2025. PMID: 40498149 Free PMC article.
-
Structural and functional roles of non-bilayer lipid phases of chloroplast thylakoid membranes and mitochondrial inner membranes.Prog Lipid Res. 2022 Apr;86:101163. doi: 10.1016/j.plipres.2022.101163. Epub 2022 Mar 26. Prog Lipid Res. 2022. PMID: 35351472 Review.
-
Lipid Polymorphism of the Subchloroplast-Granum and Stroma Thylakoid Membrane-Particles. I. 31P-NMR Spectroscopy.Cells. 2021 Sep 8;10(9):2354. doi: 10.3390/cells10092354. Cells. 2021. PMID: 34572003 Free PMC article.
-
Structural Entities Associated with Different Lipid Phases of Plant Thylakoid Membranes-Selective Susceptibilities to Different Lipases and Proteases.Cells. 2022 Aug 28;11(17):2681. doi: 10.3390/cells11172681. Cells. 2022. PMID: 36078087 Free PMC article.
-
The fluid-mosaic membrane theory in the context of photosynthetic membranes: Is the thylakoid membrane more like a mixed crystal or like a fluid?J Plant Physiol. 2020 Sep;252:153246. doi: 10.1016/j.jplph.2020.153246. Epub 2020 Jul 25. J Plant Physiol. 2020. PMID: 32777580 Review.
Cited by
-
Lipid Phase Behaviour of the Curvature Region of Thylakoid Membranes of Spinacia oleracea.Physiol Plant. 2025 May-Jun;177(3):e70289. doi: 10.1111/ppl.70289. Physiol Plant. 2025. PMID: 40525547 Free PMC article.
-
Molecular level insight into non-bilayer structure formation in thylakoid membranes: a molecular dynamics study.Photosynth Res. 2025 Jun 11;163(3):36. doi: 10.1007/s11120-025-01156-3. Photosynth Res. 2025. PMID: 40498149 Free PMC article.
References
-
- Agmon N (1995) The Grotthuss mechanism. Chemical Physics Letters 244(5): 456–462
-
- Altamura E, Albanese P, Marotta R, Milano F, Fiore M, Trotta M, Stano P, Mavelli F (2021) Chromatophores efficiently promote light‐driven ATP synthesis and DNA transcription inside hybrid multicompartment artificial cells. Proceedings of the National Academy of Sciences 118(7): e2012170118 - PMC - PubMed
-
- Aoyama M, Katayama K, Kandori H (2024) Unique hydrogen‐bonding network in a viral channelrhodopsin. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics 1865(4): 149148 - PubMed
-
- Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG (2010) An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Progress in Lipid Research 49(4): 378–389 - PubMed
-
- Bellissent‐Funel M‐C (2023) Structure and dynamics of confined water: Selected examples. Journal of Molecular Liquids 391: 123370
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

