Asthma Alleviation by Ginsenoside Rb1 via Promotion of Treg Proliferation and Inflammatory T Cell Inhibition
- PMID: 40251907
- PMCID: PMC12186593
- DOI: 10.1111/all.16551
Asthma Alleviation by Ginsenoside Rb1 via Promotion of Treg Proliferation and Inflammatory T Cell Inhibition
Abstract
Background: Regulatory T cells (Tregs) are living drugs with feasibility, tolerability, and therapeutic benefits. Although Tregs are linked to asthma prognosis through inflammation regulation, no therapeutic agents specifically designed to manage asthma by upregulating Tregs have been developed to date.
Methods: We screened a library of 250 natural products using a cytometric bead array. Among the selected candidates, gRb1 was identified for further investigation. The effects of gRb1 on Treg and Th17 populations were evaluated in mouse asthma models and human PBMCs from both healthy donors and asthma patients using flow cytometry and cytokine analysis.
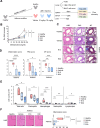
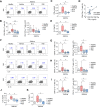
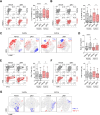
Results: In inflammatory conditions, ginsenoside Rb1 (gRb1, a major ginseng component) increased IL-10- and TGF-β-expressing Treg populations and decreased the Th17 population; activated phospho-STAT5 and NFAT1 in Tregs; inhibited NFAT1 activation in conventional T cells (Tconvs); increased Treg proliferation and Tconv-Treg differentiation, inhibiting Tconv proliferation; and reduced inflammatory cytokine secretion by Tconvs. In asthma model mice, suppression of asthma symptoms by gRb1 was associated with elevated Treg and lower Th17, Th1, and Th2 counts. gRb1 treatment of stimulated PBMCs from patients with asthma and healthy donors increased IL-10- and TGF-β-expressing Treg populations and decreased IL-17A-, IL-22-, IFN-γ-, and TNF-α-expressing T-cell populations.
Conclusions: gRb1 alleviate asthma by shifting the Treg-inflammatory T cell balance. These findings suggest a strategy for enhancing Treg activity through treatment with gRb1. This may provide a novel therapeutic approach for asthma and related disorders.
Keywords: Panax ginseng; Treg; asthma; ginsenoside Rb1; inflammation; screening.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression.J Immunol. 2011 Mar 15;186(6):3336-45. doi: 10.4049/jimmunol.1001455. Epub 2011 Feb 9. J Immunol. 2011. PMID: 21307288 Free PMC article.
-
Changes in Circulating CD44+CD62L- Treg Subsets and CD44-CD62L+ Treg Subsets Reflect the Clinical Status of Patients with Allergic Rhinitis.Int Arch Allergy Immunol. 2025;186(2):120-132. doi: 10.1159/000540536. Epub 2024 Sep 3. Int Arch Allergy Immunol. 2025. PMID: 39226877
-
Plasticity deficits of Tregs remodeling toward Th1-like and Th17-like Tregs in individuals with type 1 diabetes.J Endocrinol Invest. 2025 Jun;48(6):1495-1509. doi: 10.1007/s40618-025-02557-w. Epub 2025 Mar 3. J Endocrinol Invest. 2025. PMID: 40029535
-
Anti-IL-5 therapies for asthma.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD010834. doi: 10.1002/14651858.CD010834.pub4. Cochrane Database Syst Rev. 2022. PMID: 35838542 Free PMC article.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
References
-
- Jutel M. and Akdis C. A., “Immunological Mechanisms of Allergen‐Specific Immunotherapy,” Allergy 66 (2011): 725–732. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

