Circulating Hepatitis B Virus (HBV) RNA and Conventional Markers in Treatment-Naive Persons With HBV in Senegal
- PMID: 40251945
- PMCID: PMC12308681
- DOI: 10.1093/infdis/jiaf190
Circulating Hepatitis B Virus (HBV) RNA and Conventional Markers in Treatment-Naive Persons With HBV in Senegal
Abstract
Background: Hepatitis B virus (HBV) infection affects approximately 10% of the general population in West Africa. Circulating HBV RNA may help improve the characterization of HBV disease and prognosis. We aimed to evaluate the associations between HBV RNA and conventional biomarkers of HBV replication in the Senegalese Hepatitis B Cohort Study (SEN-B).
Methods: We included all treatment-naive, human immunodeficiency virus-negative participants of SEN-B with chronic HBV infection confirmed by a quantitative hepatitis B surface antigen (HBsAg) >0.05 IU/mL. We quantified HBV RNA, HBV DNA, and HBsAg levels and evaluated associations between those markers stratified by HBV infection phase, alanine aminotransferase level, and liver fibrosis stage.
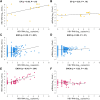
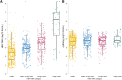
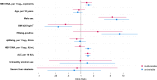
Results: Of 719 participants, 17 (2.4%) were hepatitis B e antigen (HBeAg)-positive (EP), 620 (86.2%) were classified as HBeAg-negative chronic infection (ENCI), and 82 (11.4%) were classified as HBeAg-negative chronic hepatitis (ENCH). HBV RNA was undetectable in 361 (49.8%) participants, and detectable but unquantifiable in 188 (26.1%). HBV RNA levels correlated moderately with HBV DNA levels in EP (ρ = 0.58, P = .01) and ENCH (ρ = 0.54, P < .001) and weakly in ENCI (ρ = 0.39, P < .001). HBsAg levels were only significantly correlated with HBV RNA levels in the ENCH group (ρ = -0.22, P = .05). In multivariable logistic regression, HBV RNA levels and HBV RNA to HBV DNA ratio were independently associated with significant liver fibrosis.
Conclusions: In our cohort of treatment-naive persons with HBV from Senegal, approximately 50% had undetectable HBV RNA levels. HBV RNA levels correlated with HBV DNA but not HBsAg levels in all phases of HBV infection and may provide an additional tool to assess HBV disease phase and activity.
Keywords: Africa; HBV RNA; biomarkers; hepatitis B virus; liver fibrosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. B. reports support for travel and conference participation from the CROI foundation, and speaker honoraria from Roche, paid to his institution. G. W. received research grants from Gilead Sciences and honoraria for lectures and advisory boards from Roche Diagnostics, Gilead Sciences, MSD, and ViiV, all paid to his institution. A. R. reports support to his institution for advisory boards and/or travel grants from MSD, Gilead Sciences, Pfizer, and Moderna, and an investigator-initiated trial grant from Gilead Sciences, paid to his institution and outside the submitted work. A. R. M. reports travel support from Gilead Sciences, paid to his institution. G. W. received unrestricted research grants from Gilead Sciences and Roche Diagnostics, and lecture/advisory board membership fees from Gilead Sciences, MSD, and ViiV Healthcare, all paid to his institution. All other authors reported no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- World Health Organization . Global hepatitis report 2024: action for access in low- and middle-income countries. Geneva, Switzerland: World Health Organization, 2024.
-
- European Association for the Study of the Liver . EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:370–98. - PubMed

