Conducting descriptive epidemiology and causal inference studies using observational data: A 10-point primer for stroke researchers
- PMID: 40251976
- PMCID: PMC12009273
- DOI: 10.1177/23969873251332118
Conducting descriptive epidemiology and causal inference studies using observational data: A 10-point primer for stroke researchers
Abstract
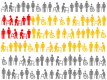
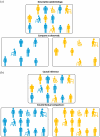
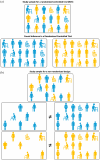
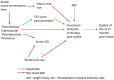
Routinely-collected health data and emerging data-linkage capabilities provide researchers and clinicians with rich opportunities to answer important research questions by conducting observational studies. We provide stroke researchers with 10 important points to consider and implement to ensure the validity and interpretability of descriptive epidemiology and causal inference studies based on observational data. We discuss different types of observational studies and biases that may arise in such studies. We review types of causal effects and the use of Target Trial emulation and Directed Acyclic Graphs to improve validity of observational studies. We also illustrate appropriate and inappropriate use of covariate adjustment for the analyses of observational studies and review the methods for estimating the effects of treatments, interventions, and exposures in causal inference studies. Finally, we provide recommendations for clinical researchers and journal manuscript reviewers in stroke domain and beyond for the appropriate use and reporting of these methods.
Keywords: Stroke; causal inference; observational data.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Designing observational studies for credible causal inference in addiction research-Directed acyclic graphs, modified disjunctive cause criterion and target trial emulation.Addiction. 2024 Jun;119(6):1125-1134. doi: 10.1111/add.16442. Epub 2024 Feb 11. Addiction. 2024. PMID: 38343103
-
Target Trial Emulation to Improve Causal Inference from Observational Data: What, Why, and How?J Am Soc Nephrol. 2023 Aug 1;34(8):1305-1314. doi: 10.1681/ASN.0000000000000152. Epub 2023 May 3. J Am Soc Nephrol. 2023. PMID: 37131279 Free PMC article. Review.
-
How to develop causal directed acyclic graphs for observational health research: a scoping review.Health Psychol Rev. 2025 Mar;19(1):45-65. doi: 10.1080/17437199.2024.2402809. Epub 2024 Sep 27. Health Psychol Rev. 2025. PMID: 39327907 Free PMC article.
-
Causal Inference About the Effects of Interventions From Observational Studies in Medical Journals.JAMA. 2024 Jun 4;331(21):1845-1853. doi: 10.1001/jama.2024.7741. JAMA. 2024. PMID: 38722735
-
Using Causal Diagrams to Improve the Design and Interpretation of Medical Research.Chest. 2020 Jul;158(1S):S21-S28. doi: 10.1016/j.chest.2020.03.011. Chest. 2020. PMID: 32658648 Review.
Cited by
-
Ultra-early computed tomography markers of haematoma expansion: Potential trial targets?Eur Stroke J. 2025 Jul 12:23969873251355938. doi: 10.1177/23969873251355938. Online ahead of print. Eur Stroke J. 2025. PMID: 40650563 Free PMC article.
-
Editorial.Eur Stroke J. 2025 Jun;10(2):306. doi: 10.1177/23969873251340774. Epub 2025 Jun 17. Eur Stroke J. 2025. PMID: 40524541 Free PMC article. No abstract available.
References
-
- Garcia-Esperon C, Bivard A, Johns H, et al. Association of endovascular thrombectomy with functional outcome in patients with acute stroke with a large ischemic core. Neurology 2022; 99: e1345–e1355. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

