Pharmacokinetics and Safety of Ceftazidime-Avibactam in Neonates and Young Infants: A Phase 2a, Multicenter Prospective Trial
- PMID: 40251980
- PMCID: PMC12117184
- DOI: 10.1093/jpids/piaf028
Pharmacokinetics and Safety of Ceftazidime-Avibactam in Neonates and Young Infants: A Phase 2a, Multicenter Prospective Trial
Abstract
Background: This phase 2a study evaluated pharmacokinetics and safety of ceftazidime-avibactam (CAZ/AVI; combination dosed as fixed 4:1 ratio) in neonates and young infants with suspected/confirmed infections due to Gram-negative pathogens requiring intravenous antibiotics.
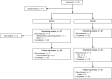
Methods: Hospitalized neonates and infants (gestational age ≥ 26 weeks to < 3 months), enrolled sequentially into 3 age cohorts, received CAZ/AVI single dose (Part A) or multiple dose every 8 h (Part B) by 2-h intravenous infusions. Infants > 28 days (Cohort 1) received CAZ/AVI 37.5 mg/kg/dose (CAZ 30 mg/kg and AVI 7.5 mg/kg). Full-term neonates ≤ 28 days (Cohort 2) and preterm neonates ≤ 28 days (Cohort 3) received 25 mg/kg/dose (CAZ 20 mg/kg and AVI 5 mg/kg). Pharmacokinetics, safety, and clinical and microbiological outcomes (Part B only) were assessed descriptively.
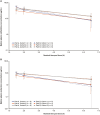
Results: Forty-six patients received CAZ/AVI, 25 in Part A and 21 in Part B. Sepsis (39.1%) and urinary tract infection (15.2%) were the predominant diagnoses. Observed drug plasma-concentration time profiles were generally similar across cohorts. Overall, 23 patients (50%) had ≥ 1 adverse event (AE), 8 patients (17.4%) had ≥ 1 serious AE (SAE), and 2 patients (4.3%) died; no SAE or death was treatment related. In Part B, ≥ 80% of patients had favorable clinical and microbiological responses.
Conclusions: Plasma exposures after single and multiple CAZ/AVI doses in neonates and young infants < 3 months old (37.5 [30/7.5] mg/kg/dose for > 28 days; 25 [20/5] mg/kg/dose for ≤ 28 days) were similar to approved doses for older children. The safety profile of CAZ/AVI was as expected based on previous observations. Study funded by Pfizer. Trial registration: NCT04126031.
Keywords: Gram-negative bacteria; antibiotic therapy; antimicrobial resistance; neonatal infection; sepsis.
Plain language summary
In neonates and young infants with suspected/confirmed infections due to Gram-negative pathogens requiring intravenous antibiotic treatment, plasma drug concentrations after single and multiple doses of ceftazidime-avibactam (CAZ/AVI) were generally similar between age cohorts. CAZ/AVI was generally well tolerated.
© The Author(s) 2025. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Conflict of interest statement
J.B. has no personal financial relationship with any company that develops, studies, or markets antibiotics. His employer, the University of California, has contracts for clinical trials and consultation with Pfizer, Merck, Shionogi, Innoviva, GSK, Melinta, and Nabriva. E.R. has received research grants to his institution from Merck, AbbVie, Shionogi, Cidara Therapeutics, and Pfizer Inc.; and is a scientific advisor and member of the speaker bureau for Gilead, Merck, Shionogi, Mundipharma, and Pfizer Inc. M.T. is a former employee of Pfizer Inc. J.Y., G.G.S., and R.E. are employees of Pfizer Inc. E.S. is an employee of Pfizer R&D UK Ltd. S.K. is an employee of Pfizer India Ltd. P.I. is an employee of Pfizer UK Ltd.
Figures
Similar articles
-
Ceftazidime-avibactam (CAZ-AVI) pharmacokinetics in critically ill patients undergoing continuous venovenous hemodiafiltration (CVVHDF).Int J Antimicrob Agents. 2025 Jan;65(1):107394. doi: 10.1016/j.ijantimicag.2024.107394. Epub 2024 Nov 23. Int J Antimicrob Agents. 2025. PMID: 39581557
-
Real-world use and treatment outcomes of ceftazidime-avibactam in gram-negative bacterial infection in Taiwan: A multicenter retrospective study.J Infect Public Health. 2025 Jun;18(6):102735. doi: 10.1016/j.jiph.2025.102735. Epub 2025 Mar 6. J Infect Public Health. 2025. PMID: 40112565
-
Renal function and its impact on the concentration of ceftazidime-avibactam: A cross-sectional study.Int J Antimicrob Agents. 2024 Dec;64(6):107351. doi: 10.1016/j.ijantimicag.2024.107351. Epub 2024 Oct 1. Int J Antimicrob Agents. 2024. PMID: 39362612
-
Pharmacokinetic drug evaluation of avibactam + ceftazidime for the treatment of hospital-acquired pneumonia.Expert Opin Drug Metab Toxicol. 2018 Mar;14(3):331-340. doi: 10.1080/17425255.2018.1434142. Epub 2018 Jan 31. Expert Opin Drug Metab Toxicol. 2018. PMID: 29373935 Review.
-
Ceftazidime/Avibactam for the Treatment of Infections in Children: A Case Series of Real-World Use.Paediatr Drugs. 2025 Jul;27(4):427-438. doi: 10.1007/s40272-025-00685-7. Epub 2025 Feb 28. Paediatr Drugs. 2025. PMID: 40019735 Review.
References
-
- Lawn JE, Blencowe H, Oza S, et al.; Lancet Every Newborn Study Group. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. https://doi.org/10.1016/S0140-6736(14)60496-7 - DOI - PubMed
-
- Fleischmann C, Reichert F, Cassini A, et al.Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106:745–752. https://doi.org/10.1136/archdischild-2020-320217 - DOI - PMC - PubMed
-
- Li J, Xiang L, Chen X, et al.Global, regional, and national burden of neonatal sepsis and other neonatal infections, 1990-2019: findings from the Global Burden of Disease Study 2019. Eur J Pediatr. 2023;182:2335–2343. https://doi.org/10.1007/s00431-023-04911-7 - DOI - PubMed
-
- Wang J, Zhang H, Yan J, Zhang T.. Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis. J Matern Fetal Neonatal Med. 2022;35:861–870. https://doi.org/10.1080/14767058.2020.1732342 - DOI - PubMed
-
- Lipworth S, Vihta K-D, Davies T, et al.Molecular epidemiology and antimicrobial resistance phenotype of paediatric bloodstream infections caused by Gram-negative bacteria. Commun Med (Lond). 2022;2:101. https://doi.org/10.1038/s43856-022-00161-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

