Transforming treatment paradigms: Focus on personalized medicine for high-grade serous ovarian cancer
- PMID: 40252048
- PMCID: PMC12432820
- DOI: 10.3322/caac.70008
Transforming treatment paradigms: Focus on personalized medicine for high-grade serous ovarian cancer
Abstract
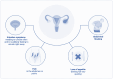
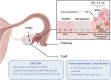
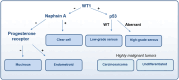
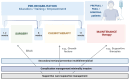
High-grade serous ovarian cancer (HGSOC) is the most common and aggressive subtype of ovarian cancer, accounting for approximately 70% of all ovarian cancer cases and contributing significantly to the high mortality rates associated with this disease. Because of the asymptomatic nature of early stage disease, most patients are diagnosed at advanced stages when the cancer has already spread into the abdominal cavity, requiring complex and intensive surgical and chemotherapeutic interventions followed by maintenance therapies. Although a minority of cases are associated with well defined genetic syndromes, specific risk factors and a clear etiology in many cases remain elusive. HGSOC tumors are characterized by a high frequency of somatic gene copy number alterations, often associated with defects in homologous recombination repair of DNA. All attempts to introduce an effective screening for HGSOC to date have been unsuccessful. This review elucidates the complexities surrounding HGSOC and encompasses its etiology, epidemiology, classification, pathogenesis, and the current array of treatment strategies. Understanding molecular underpinnings is crucial for the development of targeted therapies and personalized multimodal treatment approaches in centralized therapeutic structures. This review also examines the importance of the tumor microenvironment. In addition, the authors' objective is to underscore the critical importance of placing the patient's perspective and diversity at the forefront of therapeutic strategies, thereby fostering a genuinely participatory decision-making process and ultimately improving patient quality of life.
Keywords: diversity; high‐grade serous ovarian cancer; personalized medicine; quality of life; treatment; tumor microenvironment.
© 2025 The Author(s). CA: A Cancer Journal for Clinicians published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Kevin M. Elias reports a patent with Aspira Women's Health Inc. Marcia C. Haigis reports personal/consulting fees from Alixia and Mitoq outside the submitted work. Ioana Elena Braicu reports support for professional activities from AbbVie, AstraZeneca, GlaxoSmithKline, ImmunoGen Inc., Merck, and Myriad; and travel support from AstraZeneca outside the submitted work. The remaining authors declared no conflicts of interest. Jalid Sehouli reports research activities by Roche Pharma, AstraZeneca, Bayer, Clovis Oncology, GlaxoSmithKline, Lilly, Iqvia, Mural, and MSD; receiving honoraries by GlaxoSmithKline, PharmaMar, AstraZeneca, Clovis Oncology, Bayer, Roche Pharma, Vifor Pharma, Hexal AG, Novartis Pharma, Eisai, Esteve Pharmaceuticals, Incyte Biosciences, Phytolife Nutrition, JenaPharm, Kyowa Kirin, Oncoinvent AS, Daiichi, Medtronic Covidien, AMGEN, AbbVie, Corcept Therapeutics, Gilead Sciences, and Myriad; and consulting activities for Merck /Pfizer, PharmaMar, Clovis Oncology, AstraZeneca, Roche Pharma, GlaxoSmithKline, MSD, Eisai, Novocure, Oncoinvent, Intuitive Surgical, Seagen, Bayer Vital, Mundipharma, Sanofi‐Aventis Deutschland GmbH, Immunogen, Tubulis GmbH, Daiichi Sankyo, Bristol Myers Squibb, Karyopharm Therapeutics, and Corcept Therapeutics.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

