Research progress on ADAM28 in malignant tumors
- PMID: 40252142
- PMCID: PMC12009250
- DOI: 10.1007/s12672-025-02342-4
Research progress on ADAM28 in malignant tumors
Abstract
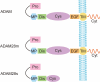
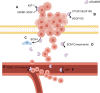
A disintegrin and metalloproteinase (ADAM) 28 belongs to the zinc-dependent metalloproteinase superfamily and has a signal sequence at its N-terminus that can direct the protein into the secretory pathway. ADAM28 is a multifunctional protein that has been shown to play a role in regulating numerous biological processes, including cell adhesion, cell fusion, membrane protein shedding, protein hydrolysis, and signaling pathway modulation. ADAM28 is highly expressed in numerous malignant tumors and plays a pivotal role in the proliferation, metastasis and drug resistance of these tumors by acting on substrates such as IGFBP-3, vWF and CTGF, thereby promoting PSGL-1/P-selectin-mediated cell adhesion. Consequently, inhibiting ADAM28 could impede tumor proliferation, metastasis and drug resistance, which suggests that ADAM28 may serve as a prognostic indicator of and potential therapeutic target for malignant tumors. In this article, the structure and function of ADAM28 and its correlation with the onset and progression of human malignant tumors are primarily examined. Additionally, the potential applications of ADAM28 in tumor research are investigated to offer a theoretical foundation and reference for the clinical diagnosis and treatment of malignant tumors.
Keywords: ADAM28; Apoptosis; Cell proliferation; Connective tissue growth factor; Drug resistance; Insulin-like growth factor-binding protein-3; Von Willebrand factor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
[Research advances on ADAM28 expression and ADAM28-mediated tumor metastasis].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014 Aug;22(4):1142-7. doi: 10.7534/j.issn.1009-2137.2014.04.049. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014. PMID: 25130845 Review. Chinese.
-
ADAM28 is overexpressed in human breast carcinomas: implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3.Cancer Res. 2006 Oct 15;66(20):9913-20. doi: 10.1158/0008-5472.CAN-06-0377. Cancer Res. 2006. PMID: 17047053
-
ADAM28 from both endothelium and gastric cancer cleaves von Willebrand Factor to eliminate von Willebrand Factor-induced apoptosis of gastric cancer cells.Eur J Pharmacol. 2021 May 5;898:173994. doi: 10.1016/j.ejphar.2021.173994. Epub 2021 Mar 3. Eur J Pharmacol. 2021. PMID: 33675784
-
Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor.J Natl Cancer Inst. 2012 Jun 20;104(12):906-22. doi: 10.1093/jnci/djs232. Epub 2012 May 25. J Natl Cancer Inst. 2012. PMID: 22636800
-
ADAM28 as a target for human cancers.Curr Pharm Des. 2009;15(20):2349-58. doi: 10.2174/138161209788682424. Curr Pharm Des. 2009. PMID: 19601836 Review.
References
-
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–30. - PubMed
-
- Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22:3855–64. - PubMed
-
- Roberts CM, Tani PH, Bridges LC, Laszik Z, Bowditch RD. MDC-L, a novel metalloprotease disintegrin cysteine-rich protein family member expressed by human lymphocytes. J Biol Chem. 1999;274:29251–9. - PubMed
-
- Mochizuki S, Shimoda M, Shiomi T, Fujii Y, Okada Y. ADAM28 is activated by MMP-7 (matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem Biophys Res Commun. 2004;315:79–84. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
