Ensitrelvir in Hospitalized Patients with SARS-CoV-2 During the Omicron Epidemic: A Single-Center Observational Study
- PMID: 40252170
- PMCID: PMC12151944
- DOI: 10.1007/s40121-025-01156-9
Ensitrelvir in Hospitalized Patients with SARS-CoV-2 During the Omicron Epidemic: A Single-Center Observational Study
Abstract
Introduction: Ensitrelvir, a novel oral 3C-like protease inhibitor targeting severe acute respiratory syndrome coronavirus 2, has been available in Japan since November 2022. This report presents patient characteristics and treatment outcomes of patients receiving ensitrelvir with comparison to remdesivir during the same period.
Methods: A single-center chart review was conducted at Rinku General Medical Center, one of four designated medical institutions for specific infectious diseases in Japan. All hospitalized patients with coronavirus disease 2019 (COVID-19) between November 2022 and August 2024 who received either ensitrelvir or remdesivir in accordance with the on-label dosage and administration were included in the review. Information on patient background, severity of COVID-19, mortality after initiation of either treatment, post-treatment virologic outcomes, and clinical outcomes were collected from electronic records. Day 28 mortality, time to discharge, and time to viral clearance were calculated with and without adjustment using the inverse probability of treatment weighting (IPTW) method.
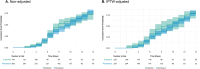
Results: During the study period, 156 patients received ensitrelvir and 337 received remdesivir as initial treatments, with average ages of 76.8 and 75.7 years, respectively. For baseline severity, 24.4% of ensitrelvir recipients and 50.7% of remdesivir recipients had moderate to severe COVID-19. All-cause mortality at day 28 was 1.9% for ensitrelvir and 5.9% for remdesivir and the hazard ratio was 0.32 (95% CI 0.09-1.07). All-cause mortality after IPTW adjustment was 3.8% and 5.7%, respectively, and the hazard ratio was 0.66 (95% CI 0.19-2.29). Time to discharge was shorter with ensitrelvir, and viral clearance was similar between groups.
Conclusion: Ensitrelvir demonstrated a low day 28 mortality, even among patients with advanced age, immunosuppressive conditions, and moderate to severe COVID-19. These findings may suggest a potential role for ensitrelvir in the treatment of hospitalized patients with COVID-19.
Trial registration: This study was registered in UMIN Clinical Trials Registry (study ID UMIN000056047).
Keywords: COVID-19; Ensitrelvir; Mortality; Remdesivir; SARS-CoV-2; Time to discharge; Time to viral clearance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Masaya Yamato has received lecture fees from, and serves as an advisor for, Shionogi & Co., Ltd. Masahiro Kinoshita, Yuki Yoshida, and Takuhiro Sonoyama are employees of Shionogi & Co., Ltd.. Yudai Yamamoto and Rie Izuhara have no conflicts of interest to disclose. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki, and the Ethical Guidelines for Life Sciences and Medical Research Involving Human Subjects. The research protocol was reviewed and approved by the Rinku General Medical Center Clinical Research Ethics Committee (2-23 Rinku Ourai-Kita, Izumisano, Osaka 598-8577, Japan; protocol ID 2024FY-I-003; date of approval 20 August 2024). Patient consent was acquired using an opt-out procedure and no data were included that would allow identification of individual patients.
Figures

Similar articles
-
Ensitrelvir in patients with SARS-CoV-2: A retrospective chart review.J Infect Chemother. 2024 Sep;30(9):946-950. doi: 10.1016/j.jiac.2024.02.015. Epub 2024 Feb 15. J Infect Chemother. 2024. PMID: 38367932
-
A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part.Antimicrob Agents Chemother. 2022 Oct 18;66(10):e0069722. doi: 10.1128/aac.00697-22. Epub 2022 Sep 13. Antimicrob Agents Chemother. 2022. PMID: 36098519 Free PMC article. Clinical Trial.
-
Ensitrelvir as a novel treatment option for mild-to-moderate COVID-19: a narrative literature review.Ther Adv Infect Dis. 2025 Apr 11;12:20499361251321724. doi: 10.1177/20499361251321724. eCollection 2025 Jan-Dec. Ther Adv Infect Dis. 2025. PMID: 40292087 Free PMC article. Review.
-
Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19.Infect Dis Ther. 2024 Aug;13(8):1821-1833. doi: 10.1007/s40121-024-01010-4. Epub 2024 Jun 28. Infect Dis Ther. 2024. PMID: 38941067 Free PMC article.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD014962. doi: 10.1002/14651858.CD014962. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jan 25;1:CD014962. doi: 10.1002/14651858.CD014962.pub2. PMID: 34350582 Free PMC article. Updated.
References
-
- Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022;94(4):1738–44. - PubMed
-
- Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–52. - PMC - PubMed
-
- Khadela A, Soni S, Megha K, et al. A review on the impact of the SARS-CoV-2 Omicron subvariant on elderly patients with diverse co-morbidities. Biologics. 2023;3:138–57.
LinkOut - more resources
Full Text Sources
Miscellaneous

