CKAP5 deficiency induces premature ovarian insufficiency
- PMID: 40252251
- PMCID: PMC12032925
- DOI: 10.1016/j.ebiom.2025.105718
CKAP5 deficiency induces premature ovarian insufficiency
Abstract
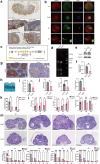
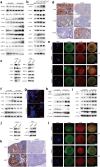
Background: Premature ovarian insufficiency (POI) is characterized by ovarian dysfunction that develops from diminished ovarian reserve (DOR). The exact aetiology of POI remains poorly understood. This study aims to elucidate the role of CKAP5 in the regulation of ovarian function and fertility.
Methods: Bulk RNA sequencing of granulosa cells was conducted in the control group and in the patients with DOR to screen for candidate genes, which were further validated by gene burden analysis in a next-generation sequencing cohort of POI and control individuals. Additionally, ovarian reserve was evaluated in heterozygous Ckap5 knockout mice, alongside the ovarian and oocyte single-cell transcriptome analysis. The regulatory mechanism of CKAP5 was studied through in vivo and in vitro experiments.
Findings: CKAP5 was identified as a key hub gene associated with ovarian ageing. Heterozygous Ckap5 knockout mice exhibited a POI-like phenotype, characterized by a reduced primordial follicle pool and accelerated follicular atresia. CKAP5 promotes autophagy via ATG7 and simultaneously supports DNA damage repair through the ATM. Finally, a variant in CKAP5 (NM_0001008938.4, c.630 + 7_630 + 11delCAAAA) was identified in patients with POI, resulting in protein truncation and loss of function.
Interpretation: CKAP5 deficiency induces premature ovarian insufficiency in both humans and mice.
Funding: The National Key R&D Program of China (2017YFC1001100), the National Natural Science Foundation of China (81501248, 81471453 and 81801295), the Health Research Project of Hunan Provincial Health Commission (W20243018), the Science and Technology Innovation Program of Hunan Province (2021RC3031), the National Natural Science Foundation of Hunan Province (2022JJ30066), the Scientific Research Program of Hunan Provincial Health Commission (202205033471 and 21B0058), the Open Research Fund of Hunan Provincial Key Laboratory of Regional Hereditary Birth Defects Prevention and Control (HPKL2023013).
Keywords: Apoptosis; Autophagy; CKAP5; DNA damage repair; Premature ovary insufficiency.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures



Similar articles
-
Single-nucleus and spatial transcriptomics of paediatric ovary: Molecular insights into the dysregulated signalling pathways underlying premature ovarian insufficiency in classic galactosemia.Clin Transl Med. 2024 Oct;14(10):e70043. doi: 10.1002/ctm2.70043. Clin Transl Med. 2024. PMID: 39440457 Free PMC article.
-
Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency.Mol Hum Reprod. 2016 Jun;22(6):384-96. doi: 10.1093/molehr/gaw023. Epub 2016 Mar 9. Mol Hum Reprod. 2016. PMID: 26965313 Free PMC article.
-
[Homozygous Variant of FANCM of the Fanconi Anemia Pathway Causes Premature Ovarian Insufficiency: Investigation of the Pathogenic Mechanism].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):559-565. doi: 10.12182/20240560207. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948269 Free PMC article. Chinese.
-
A kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence.FASEB J. 2021 Aug;35(8):e21753. doi: 10.1096/fj.202100756R. FASEB J. 2021. PMID: 34233068 Review.
-
Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell.J Mol Med (Berl). 2021 May;99(5):637-650. doi: 10.1007/s00109-021-02055-5. Epub 2021 Feb 27. J Mol Med (Berl). 2021. PMID: 33641066 Review.
Cited by
-
Molecular Guardians of Oocyte Maturation: A Systematic Review on TUBB8, KIF11, and CKAP5 in IVF Outcomes.Int J Mol Sci. 2025 Jul 2;26(13):6390. doi: 10.3390/ijms26136390. Int J Mol Sci. 2025. PMID: 40650169 Free PMC article. Review.
-
Stage-specific autophagy dynamics in reproductive processes and associated disorders.Front Cell Dev Biol. 2025 Jul 28;13:1639691. doi: 10.3389/fcell.2025.1639691. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40791986 Free PMC article. Review.
References
-
- Webber L., Davies M., Anderson R., et al. European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. - PubMed
-
- Golezar S., Ramezani Tehrani F., Khazaei S., Ebadi A., Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403–411. - PubMed
-
- Jiao X., Ke H., Qin Y., Chen Z.J. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

