Endometriosis and adenomyosis unveiled through single-cell glasses
- PMID: 40253075
- PMCID: PMC12282335
- DOI: 10.1016/j.ajog.2024.08.043
Endometriosis and adenomyosis unveiled through single-cell glasses
Abstract
Single-cell technologies are expanding our understanding of endometriosis and adenomyosis, which are sister disorders of the uterine endometrium that contain similar complements of lesion cell types but in different locations-outside and inside the uterus, respectively. Both diseases cause significant morbidity and impaired quality of life among those affected, and current therapies mitigate most of the symptoms although with highly variable efficacy, duration of effect, and frequent intolerable side effects. Thus, there is a pressing need for transformative approaches and to develop individualized therapies for the variety of presentations of endometriosis and adenomyosis symptoms and the heterogeneity of lesion types, both histologically and architecturally. Single-cell technologies are transforming the understanding of human physiology and pathophysiology in the reproductive system and beyond. This manuscript reviews the clinical characteristics of endometriosis and adenomyosis and the recent studies focused on eutopic endometrium and ectopic lesions at single-cell resolution, the myriad of cell types and subtypes, cell-cell communications, signaling pathways, applications for novel drug discovery and therapeutic approaches, and challenges and opportunities that accompany this type of research. Key take-home messages from the studies reviewed herein include the following: We conclude the review with an eye to the future-what Alice might see beyond the single-cell looking glass that connects endometrium and endometrial disorders with the trillions of cells of other tissues and organs in health and disease throughout the human body and the opportunities for novel diagnostic modalities and drug discovery for endometriosis, adenomyosis, and related uterine and inflammatory conditions.
Keywords: adenomyosis; biomarkers; cell types; drug discovery; endometriosis; inflammation; precision medicine; sequencing; signaling pathways; single cell.
Copyright © 2024 Elsevier Inc. All rights reserved.
Figures

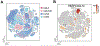

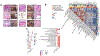
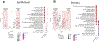
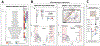



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

