Intranasal insulin for improving cognitive function in multiple sclerosis
- PMID: 40253245
- PMCID: PMC12418434
- DOI: 10.1016/j.neurot.2025.e00581
Intranasal insulin for improving cognitive function in multiple sclerosis
Abstract
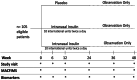
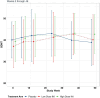
Cognitive impairment is common in people with multiple sclerosis (PwMS). There is an urgent need to identify/develop novel therapies that can help cognitive function in MS. Insulin is critical for helping with regulation of multiple CNS functions, including learning and memory. Insulin administrated intranasally has shown to improve memory and learning in healthy people and in those with some neurodegenerative disorders. Hence, there was rationale for investigating intranasal insulin in PwMS who experience cognitive impairment. We completed a phase Ib/II, randomized, double-blind, placebo-controlled trial; participants were randomized in a 1:1:1 fashion, stratified by relapsing versus progressive MS, to intranasal insulin 10 international units (IU) twice a day, 20 IU twice a day, or placebo for 24 weeks. One-hundred and five PwMS were enrolled, 69 of whom had at least one follow up visit during the active treatment phase of the trial (baseline to week 24). The cohort's mean age was 52.4 ± 9.7years, 62 % were female, and ∼60 % had relapsing-remitting MS. The most common side effects amongst treatment groups included headache, rhinorrhea, and dizziness. There were 13 SAEs which were not deemed study drug related; there were no deaths. The main clinical outcome measure, SDMT, did not demonstrate any difference between intranasal insulin and placebo. Similar findings were noted for all secondary outcome measures. Intranasal insulin appeared safe and well-tolerated in PwMS. However, it was not superior to placebo in any of the clinical outcome measures assessed, which could have been impacted by the duration of the trial, small sample size for a three-arm trial design, data missingness (particularly during COVID-19), outcome measure insensitivity to change, baseline cognitive reserve, or other factors. Nonetheless, intranasally-administered therapeutics may be of interest to develop further as a way to get across the blood brain barrier.
Keywords: Clinical trials; Cognitive dysfunction; Insulin; Intranasal treatments; Multiple sclerosis.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures
References
-
- Benedict R.H., Cookfair D., Gavett R., Gunther M., Munschauer F., Garg N., et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006 Jul;12(4):549–558. - PubMed
-
- DeLuca J. In: Multiple sclerosis: understanding the cognitive challenges. LaRocca N., Kalb R., editors. Demos Health; New York: 2006. What we know about cognitive changes in multiple sclerosis; pp. 17–40.
-
- Rao S. Cognitive function in patients with multiple sclerosis: impairment and treatment. IJMSC. 2004;1:9–22.
-
- Ruet A., Deloire M., Hamel D., Ouallet J.C., Petry K., Brochet B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260(3):776–784. - PubMed
-
- Strober L.B., Christodoulou C., Benedict R.H., Westervelt H.J., Melville P., Scherl W.F., et al. Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler. 2012;18(5):647–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical