The first trimester human placenta responds to Zika virus infection inducing an interferon (IFN) and antiviral interferon stimulated gene (ISG) response
- PMID: 40253335
- PMCID: PMC12008946
- DOI: 10.1186/s12985-025-02729-3
The first trimester human placenta responds to Zika virus infection inducing an interferon (IFN) and antiviral interferon stimulated gene (ISG) response
Abstract
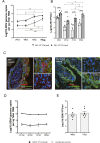
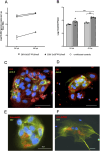
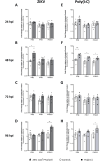
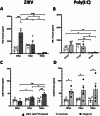
Background: Zika virus (ZIKV) is a positive-strand RNA virus of the Flaviviridae family. Maternal ZIKV infection during pregnancy can spread to the placenta and fetus causing severe neurological defects and infants born with microcephaly. Here, we investigated ZIKV infection and the cellular innate antiviral immune response in first trimester human placental explant cultures and isolated primary villus cytotrophoblasts (CTBs).
Methods: Placentas were obtained with informed consent from women undergoing elective pregnancy termination and either cultured as placental explants or used to isolate primary CTBs. Explants and CTBs were both infected with ZIKV (PRVABC59), and samples evaluated for infection by qRT-PCR, viral plaque and ELISA assays, and immunohistochemical or immunocytochemical staining.
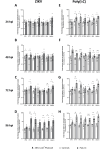
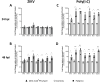
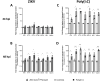
Results: We demonstrate robust infection and production of ZIKV in placental explant and CTB cultures. Both displayed delayed upregulation of interferons (IFN), most notably IFNβ and IFNλ2/3, and a panel of interferon stimulated genes (ISG) (IFI6, IFIT1, IFIT2, IFITM1, ISG15, MX1, RSAD). Stimulation of explants and CTBs with the dsRNA mimic poly(I: C), caused immediate IFN and ISG upregulation, demonstrating the first trimester placenta is innate immune competent. This suggests that either ZIKV blocks the early innate response, or the placental response is inherently hindered.
Conclusion: Together these data show that first trimester placenta is susceptible to ZIKV infection which induces a delayed type III IFN antiviral response. This delay likely creates an environment favourable to ZIKV replication and dissemination across the early gestation placenta to fetal tissue, causing pathologies associated with congenital ZIKV syndrome.
Keywords: First trimester pregnancy; Interferon; Interferon lambda; Interferon stimulated genes; Placenta; Trophoblast; Zika virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent: All placentas were obtained with written informed consent from women undergoing elective pregnancy terminations at the Pregnancy Advisory Centre (PAC), Woodville, South Australia. Ethics approval was granted by the Queen Elizabeth Hospital Human Research Ethics Committee (HREC/16/TQEH/33). Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Zika virus-induced fetal demise is triggered by strain- and dose-specific RLR-driven activation of the interferon response in the decidua, placenta, and fetus in Ifnar1-/- mice.J Virol. 2025 Jun 17;99(6):e0066625. doi: 10.1128/jvi.00666-25. Epub 2025 May 22. J Virol. 2025. PMID: 40401980 Free PMC article.
-
Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface.J Virol. 2017 Jan 31;91(4):e01905-16. doi: 10.1128/JVI.01905-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974560 Free PMC article.
-
Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection.Cell Host Microbe. 2016 May 11;19(5):705-12. doi: 10.1016/j.chom.2016.03.008. Epub 2016 Apr 5. Cell Host Microbe. 2016. PMID: 27066743 Free PMC article.
-
Zika virus infection of first-trimester human placentas: utility of an explant model of replication to evaluate correlates of immune protection ex vivo.Curr Opin Virol. 2017 Dec;27:48-56. doi: 10.1016/j.coviro.2017.11.008. Curr Opin Virol. 2017. PMID: 29172071 Free PMC article. Review.
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

