Disruption-induced changes in syntrophic propionate and acetate oxidation: flocculation, cell proximity, and microbial activity
- PMID: 40253350
- PMCID: PMC12008871
- DOI: 10.1186/s13068-025-02644-3
Disruption-induced changes in syntrophic propionate and acetate oxidation: flocculation, cell proximity, and microbial activity
Abstract
Background: Syntrophic propionate- and acetate-oxidising bacteria (SPOB and SAOB) play a crucial role in biogas production, particularly under high ammonia conditions that are common in anaerobic degradation of protein-rich waste streams. These bacteria rely on close interactions with hydrogenotrophic methanogens to facilitate interspecies electron transfer and maintain thermodynamic feasibility. However, the impact of mixing-induced disruption of these essential syntrophic interactions in biogas systems remains largely unexplored. This study investigates how magnetic stirring and orbital shaking influence degradation dynamics, microbial community composition, and gene expression in syntrophic enrichment communities under high-ammonia conditions.
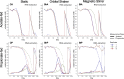
Results: Stirring significantly delayed the initiation of propionate degradation in one culture and completely inhibited it in the other two parallel cultures, whereas acetate degradation was less affected. Computational fluid dynamics modelling revealed that stirring generated higher shear rates (~ 20 s-1) and uniform cell distribution, while shaking led to lower shear rates and cell accumulation at the bottom of the culture bottle. Visual observations confirmed that stirring inhibited floc formation, while shaking promoted larger flocs compared to the static control condition, which formed smaller flocs and a sheet-like biofilm. Microbial community analysis identified substrate type and degradation progress as primary drivers of community structure, with motion displaying minimal influence. However, metatranscriptomic analysis revealed that motion-induced gene downregulation was associated with motility, surface sensing, and biofilm formation in SAOB and another bacterial species expressing genes for the glycine synthase reductase pathway. Stirring also suppressed oxalate-formate antiporter expression in SPOB, suggesting its dependence on spatial proximity for this energy-conserving mechanism. The strongest gene expression changes of stirring were observed in methanogens, indicating a coupling of the first and last steps of hydrogenotrophic methanogenesis, likely an adaptive strategy for efficient energy conservation. Other downregulated genes included ferrous iron transporters and electron transfer-associated enzymes.
Conclusions: This study highlights that stirring critically disrupts the initial syntrophic connection between SPOB and methanogens, whereas SAOB communities exhibit greater tolerance to shear stress and disruptive conditions that inhibits aggregate formation. These findings emphasize the importance of carefully managing mixing regimes, especially when attempting to reactivate ammonia-tolerant syntrophic propionate degraders in biogas systems experiencing rapid propionate accumulation under high-ammonia conditions.
Keywords: Anaerobic digestion; Computational fluid dynamics; Flocculation; Interspecies electron transfer; Methanogens; Mixing; Syntrophic acetate-oxidizing bacteria; Syntrophic propionate-oxidizing bacteria.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Catabolism and interactions of syntrophic propionate- and acetate oxidizing microorganisms under mesophilic, high-ammonia conditions.Front Microbiol. 2024 Jun 5;15:1389257. doi: 10.3389/fmicb.2024.1389257. eCollection 2024. Front Microbiol. 2024. PMID: 38933034 Free PMC article.
-
Impact of additives on syntrophic propionate and acetate enrichments under high-ammonia conditions.Appl Microbiol Biotechnol. 2024 Aug 7;108(1):433. doi: 10.1007/s00253-024-13263-7. Appl Microbiol Biotechnol. 2024. PMID: 39110235 Free PMC article.
-
Syntrophic entanglements for propionate and acetate oxidation under thermophilic and high-ammonia conditions.ISME J. 2023 Nov;17(11):1966-1978. doi: 10.1038/s41396-023-01504-y. Epub 2023 Sep 7. ISME J. 2023. PMID: 37679429 Free PMC article.
-
Syntrophic propionate-oxidizing bacteria in methanogenic systems.FEMS Microbiol Rev. 2022 Mar 3;46(2):fuab057. doi: 10.1093/femsre/fuab057. FEMS Microbiol Rev. 2022. PMID: 34875063 Free PMC article. Review.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
References
-
- Anukam A, Mohammadi A, Naqvi M, Granström K. A review of the chemistry of anaerobic digestion: methods of accelerating and optimizing process efficiency. Processes. 2019;7:504.
-
- Giduthuri AT, Ahring BK. Current status and prospects of valorizing organic waste via arrested anaerobic digestion: production and separation of volatile fatty acids. Fermentation. 2023;9:13.
-
- Scarlat N, Dallemand J-F, Fahl F. Biogas: developments and perspectives in Europe. Renew Energy. 2018;129:457–72.
-
- O’Connor J, Mickan BS, Rinklebe J, Song H, Siddique KHM, Wang H, et al. Environmental implications, potential value, and future of food-waste anaerobic digestate management: a review. J Environ Manage. 2022;318: 115519. - PubMed
-
- EurObserv’ER. 21st annual overview barometer. 2022. https://www.eurobserv-er.org/21st-annual-overview-barometer/. Accessed 10 July 2023.
Grants and funding
LinkOut - more resources
Full Text Sources
