Structural basis for Polθ-helicase DNA binding and microhomology-mediated end-joining
- PMID: 40253368
- PMCID: PMC12009414
- DOI: 10.1038/s41467-025-58441-x
Structural basis for Polθ-helicase DNA binding and microhomology-mediated end-joining
Abstract
DNA double-strand breaks (DSBs) present a critical threat to genomic integrity, often precipitating genomic instability and oncogenesis. Repair of DSBs predominantly occurs through homologous recombination (HR) and non-homologous end joining (NHEJ). In HR-deficient cells, DNA polymerase theta (Polθ) becomes critical for DSB repair via microhomology-mediated end joining (MMEJ), also termed theta-mediated end joining (TMEJ). Thus, Polθ is synthetically lethal with BRCA1/2 and other HR factors, underscoring its potential as a therapeutic target in HR-deficient cancers. However, the molecular mechanisms governing Polθ-mediated MMEJ remain poorly understood. Here we present a series of cryo-electron microscopy structures of the Polθ helicase domain (Polθ-hel) in complex with DNA containing different 3'-ssDNA overhangs. The structures reveal the sequential conformations adopted by Polθ-hel during the critical phases of DNA binding, microhomology searching, and microhomology annealing. The stepwise conformational changes within the Polθ-hel subdomains and its functional dimeric state are pivotal for aligning the 3'-ssDNA overhangs, facilitating the microhomology search and subsequent annealing necessary for DSB repair via MMEJ. Our findings illustrate the essential molecular switches within Polθ-hel that orchestrate the MMEJ process in DSB repair, laying the groundwork for the development of targeted therapies against the Polθ-hel.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: X.S.C. is a cofounder of Recombination Therapeutics, LLC. R.T.P. is a cofounder and CSO of Recombination Therapeutics, LLC. The remaining authors declare no competing interests.
Figures

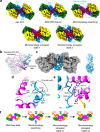

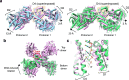
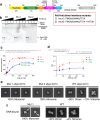
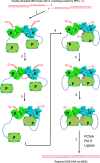
Update of
-
Structural Basis for Polθ-Helicase DNA Binding and Microhomology-Mediated End-Joining.bioRxiv [Preprint]. 2024 Jun 7:2024.06.07.597860. doi: 10.1101/2024.06.07.597860. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 19;16(1):3725. doi: 10.1038/s41467-025-58441-x. PMID: 38895274 Free PMC article. Updated. Preprint.
Similar articles
-
Structural Basis for Polθ-Helicase DNA Binding and Microhomology-Mediated End-Joining.bioRxiv [Preprint]. 2024 Jun 7:2024.06.07.597860. doi: 10.1101/2024.06.07.597860. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 19;16(1):3725. doi: 10.1038/s41467-025-58441-x. PMID: 38895274 Free PMC article. Updated. Preprint.
-
Molecular basis of microhomology-mediated end-joining by purified full-length Polθ.Nat Commun. 2019 Sep 27;10(1):4423. doi: 10.1038/s41467-019-12272-9. Nat Commun. 2019. PMID: 31562312 Free PMC article.
-
Polymerase theta is a synthetic lethal target for killing Epstein-Barr virus lymphomas.J Virol. 2024 Jul 23;98(7):e0057224. doi: 10.1128/jvi.00572-24. Epub 2024 Jun 11. J Virol. 2024. PMID: 38860782 Free PMC article.
-
DNA polymerase theta (Polθ) - an error-prone polymerase necessary for genome stability.Curr Opin Genet Dev. 2020 Feb;60:119-126. doi: 10.1016/j.gde.2020.02.017. Epub 2020 Apr 14. Curr Opin Genet Dev. 2020. PMID: 32302896 Free PMC article. Review.
-
Microhomology-Mediated End-Joining Chronicles: Tracing the Evolutionary Footprints of Genome Protection.Annu Rev Cell Dev Biol. 2024 Oct;40(1):195-218. doi: 10.1146/annurev-cellbio-111822-014426. Epub 2024 Sep 21. Annu Rev Cell Dev Biol. 2024. PMID: 38857538 Review.
Cited by
-
Making 3' ends meet.Nat Struct Mol Biol. 2025 Jun;32(6):959-960. doi: 10.1038/s41594-025-01578-6. Nat Struct Mol Biol. 2025. PMID: 40404983 No abstract available.
References
-
- Ramsden, D. A., Carvajal-Garcia, J. & Gupta, G. P. Mechanism, cellular functions and cancer roles of polymerase-theta-mediated DNA end joining. Nat. Rev. Mol. Cell Biol.23, 125–140 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI150524/AI/NIAID NIH HHS/United States
- R35 GM152198/GM/NIGMS NIH HHS/United States
- R01AI150524/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R35GM152198/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources
Miscellaneous

