Multivariate covalent organic frameworks with tailored electrostatic potential promote nitrate electroreduction to ammonia in acid
- PMID: 40253373
- PMCID: PMC12009421
- DOI: 10.1038/s41467-025-59052-2
Multivariate covalent organic frameworks with tailored electrostatic potential promote nitrate electroreduction to ammonia in acid
Abstract
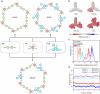
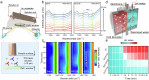
The direct synthesis of ammonia from nitrate (NO3-) reduction in acid is a promising approach for industrialization. However, the difficulty arises from the intense competition with the inevitable hydrogen evolution reaction, which is favoured due to the overwhelming protons (H+). Here, we systematically explore and rationally optimize the microenvironment using multivariate covalent organic frameworks (COFs) as catalyst adlayers to promote the nitrate-to-ammonia conversion in acid. With the application of tailored positive electrostatic potential generated over the multivariate COFs, both the mass transfer of NO3- and H+ are regulated via appropriate electrostatic interactions, thus realizing the priority of NO3RR with respect to HER or NO3--to-NO2-. As a result, an NH3 yield rate of 11.01 mmol h-1 mg-1 and a corresponding Faradaic efficiency of 91.0% are attained, and solid NH4Cl with a high purity of 96.2% is directly collected in acid; therefore, this method provides a practical approach for economically valorising wastewater into valuable ammonia.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
B-doped MoS2 for nitrate electroreduction to ammonia.J Colloid Interface Sci. 2023 Jan;629(Pt A):950-957. doi: 10.1016/j.jcis.2022.09.049. Epub 2022 Sep 13. J Colloid Interface Sci. 2023. PMID: 36152619
-
Tuning the Oxidation State of Cu Electrodes for Selective Electrosynthesis of Ammonia from Nitrate.ACS Appl Mater Interfaces. 2021 Nov 10;13(44):52469-52478. doi: 10.1021/acsami.1c10946. Epub 2021 Nov 1. ACS Appl Mater Interfaces. 2021. PMID: 34723479
-
Molecular Engineering of Hydrogen-Bonded Organic Framework for Enhanced Nitrate Electroreduction to Ammonia.Nano Lett. 2024 Jul 17;24(28):8687-8695. doi: 10.1021/acs.nanolett.4c02030. Epub 2024 Jul 8. Nano Lett. 2024. PMID: 38973752
-
Efficient Electroreduction of Nitrate into Ammonia at Ultralow Concentrations Via an Enrichment Effect.Adv Mater. 2022 Sep;34(36):e2204306. doi: 10.1002/adma.202204306. Epub 2022 Aug 7. Adv Mater. 2022. PMID: 35839314
-
Progress and perspectives in the electroreduction of low-concentration nitrate for wastewater management.iScience. 2024 Dec 21;28(1):111687. doi: 10.1016/j.isci.2024.111687. eCollection 2025 Jan 17. iScience. 2024. PMID: 39877065 Free PMC article. Review.
Cited by
-
Maximized atom utilization in a high-entropy metallene via single atom alloying for boosted nitrate electroreduction to ammonia.Nat Commun. 2025 Aug 25;16(1):7915. doi: 10.1038/s41467-025-63317-1. Nat Commun. 2025. PMID: 40855078 Free PMC article.
References
-
- Ashida, Y., Arashiba, K., Nakajima, K. & Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature568, 536–540 (2019). - PubMed
-
- Liu, S. et al. Awakening (220) as one more active facet of PtMo alloy via single-atom doping to boost ammonia electrooxidation in direct ammonia fuel cell. Adv. Funct. Mater.33, 2306204 (2023).
-
- Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev.109, 2209–2244 (2009). - PubMed
-
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal.2, 377–380 (2019).
-
- Cheng, Q. et al. High-entropy alloys for accessing hydrogen economy via sustainable production of fuels and direct application in fuel cells. Rare Met.42, 3553–3569 (2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

