SNAP25-dependent membrane trafficking of the Kv1.5 channel regulates the onset of atrial fibrillation
- PMID: 40253375
- PMCID: PMC12009440
- DOI: 10.1038/s41467-025-59096-4
SNAP25-dependent membrane trafficking of the Kv1.5 channel regulates the onset of atrial fibrillation
Abstract
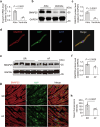
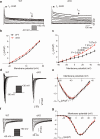
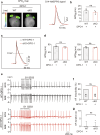
Synaptosomal-associated protein 25 kDa (SNAP25) is essential for vesicular trafficking and protein docking at presynaptic membranes in the nervous system, yet its role in the heart remains poorly understood. Here, we show an unrecognized function of SNAP25, which is selectively expressed in the atria, in regulating atrial electrical remodeling and the onset of atrial fibrillation (AF). SNAP25 protein is downregulated in the atria of AF patients. Cardiomyocyte-specific knockout of SNAP25 in male mice significantly shortens the atrial effective refractory period and action potential duration (APD), increasing susceptibility to AF, which is attributed to elevated Kv1.5 current and membrane expression. Blocking Kv1.5 channels effectively restores atrial APD and reduces AF incidence. Mechanistically, SNAP25 deficiency reduces the internalization of Kv1.5 from the cell surface membrane to early endosomes. In human iPSC-derived atrial cardiomyocytes, SNAP25 deficiency similarly elevates arrhythmic activity and accelerates repolarization. In conclusion, this study reveals that SNAP25 regulates AF susceptibility by controlling the trafficking of the atrial-specific Kv1.5 channel, highlighting SNAP25 as a promising therapeutic target for atrial arrhythmias.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Microtubule polymerization state and clathrin-dependent internalization regulate dynamics of cardiac potassium channel: Microtubule and clathrin control of KV1.5 channel.J Mol Cell Cardiol. 2020 Jul;144:127-139. doi: 10.1016/j.yjmcc.2020.05.004. Epub 2020 May 20. J Mol Cell Cardiol. 2020. PMID: 32445844
-
Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation.Basic Res Cardiol. 2015 Sep;110(5):505. doi: 10.1007/s00395-015-0505-6. Epub 2015 Jul 11. Basic Res Cardiol. 2015. PMID: 26162324 Free PMC article.
-
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.Circ Res. 2014 Mar 14;114(6):982-92. doi: 10.1161/CIRCRESAHA.114.302711. Epub 2014 Feb 7. Circ Res. 2014. PMID: 24508725 Free PMC article.
-
[Biology of the substrate of atrial fibrillation].Biol Aujourdhui. 2012;206(1):5-9. doi: 10.1051/jbio/2012004. Epub 2012 Apr 3. Biol Aujourdhui. 2012. PMID: 22463991 Review. French.
-
The molecular and functional identities of atrial cardiomyocytes in health and disease.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1882-93. doi: 10.1016/j.bbamcr.2015.11.025. Epub 2015 Nov 24. Biochim Biophys Acta. 2016. PMID: 26620800 Review.
References
-
- Brundel, B. et al. Atrial fibrillation. Nat. Rev. Dis. Prim.8, 21 (2022). - PubMed
-
- Baman, J. R. & Passman, R. S. Atrial Fibrillation. Jama325, 2218 (2021). - PubMed
-
- Michaud, G. F. & Stevenson, W. G. Atrial Fibrillation. N. Engl. J. Med.384, 353–361 (2021). - PubMed
-
- Seiffge, D. J. et al. Secondary stroke prevention in people with atrial fibrillation: treatments and trials. Lancet Neurol.23, 404–417 (2024). - PubMed
-
- Spatz, E. S. & Herrin, J. Screening for atrial fibrillation to prevent stroke: increasing enthusiasm but outcomes still lag. Eur. Heart J.44, 205–207 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

