Temozolomide promotes glioblastoma stemness expression through senescence-associated reprogramming via HIF1α/HIF2α regulation
- PMID: 40253386
- PMCID: PMC12009364
- DOI: 10.1038/s41419-025-07617-w
Temozolomide promotes glioblastoma stemness expression through senescence-associated reprogramming via HIF1α/HIF2α regulation
Abstract
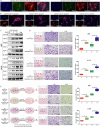
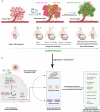
A critical challenge in glioblastoma multiforme (GBM) treatment is that tumors recurring after temozolomide (TMZ) therapy become more malignant, exhibiting increased invasiveness and stemness compared to the primary tumor. However, the underlying mechanisms remain unclear. While the majority of GBM cells are eradicated by TMZ, a subset enters cell cycle arrest, adopts a senescence-associated secretory phenotype (SASP), and activates senescence-related signaling pathways. These cells eventually escape senescence, re-enter the cell cycle, and form aggregates exhibiting stem-like characteristics such as elevated stemness marker expression, enhanced colony formation, increased invasiveness, and resistance to chemotherapy. Furthermore, these aggregates promote the invasion and chemotherapy resistance of surrounding cells. Gene Set Enrichment Analysis (GSEA) and KEGG pathway analysis of miRNA and mRNA sequences revealed activation of hallmark hypoxia and HIF1 signaling pathways. The study demonstrated that HIF1α and HIF2α expression fluctuates during and after TMZ treatment. Knockout of HIF1α and HIF2α in GBM cells exposed to TMZ reduced the formation of senescent cells and stem-like aggregates. These findings challenge the efficacy of TMZ therapy by highlighting its role in inducing the process of cellular senescence, thereby contributing to the enhanced stemness and malignancy of recurrent GBM. The regulatory roles of HIF1α and HIF2α are emphasized, underscoring the necessity of preventing senescent cell formation and inhibiting HIF1α/HIF2α expression to improve therapeutic outcomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest. Ethics: This study was done in compliance with the principles of the Declaration of Helsinki. Ethical related to GBM tissues were approved by the ethics committee of Chongqing General Hospital (No. KY S2022-094-01) and the informed consent was obtained from the patients. All the animal procedures were approved by the ethics committee of ChongQing Medical University (No. IACUC-CQMU-2023-0167).
Figures





Similar articles
-
The HIF1α/HIF2α-miR210-3p network regulates glioblastoma cell proliferation, dedifferentiation and chemoresistance through EGF under hypoxic conditions.Cell Death Dis. 2020 Nov 18;11(11):992. doi: 10.1038/s41419-020-03150-0. Cell Death Dis. 2020. PMID: 33208727 Free PMC article.
-
HIF1α/HIF2α-Sox2/Klf4 promotes the malignant progression of glioblastoma via the EGFR-PI3K/AKT signalling pathway with positive feedback under hypoxia.Cell Death Dis. 2021 Mar 24;12(4):312. doi: 10.1038/s41419-021-03598-8. Cell Death Dis. 2021. PMID: 33762574 Free PMC article.
-
In Silico and In Vitro Study of mRNA Biomarkers for Glioblastoma Multiforme Resistance to Temozolomide (TMZ): The Association with Stemness.Asian Pac J Cancer Prev. 2024 Dec 1;25(12):4435-4446. doi: 10.31557/APJCP.2024.25.12.4435. Asian Pac J Cancer Prev. 2024. PMID: 39733437 Free PMC article.
-
An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective.Int J Mol Sci. 2018 Mar 17;19(3):889. doi: 10.3390/ijms19030889. Int J Mol Sci. 2018. PMID: 29562589 Free PMC article. Review.
-
Drug resistance and cancer stem cells: the shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α.Clin Exp Pharmacol Physiol. 2017 Feb;44(2):153-161. doi: 10.1111/1440-1681.12693. Clin Exp Pharmacol Physiol. 2017. PMID: 27809360 Review.
References
-
- Chen C, Jing WQ, Chen Y, Wang GY, Abdalla M, Gao L, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. 2022;14:eabn1128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

