Age-dependent cortical overconnectivity in Shank3 mice is reversed by anesthesia
- PMID: 40253406
- PMCID: PMC12009330
- DOI: 10.1038/s41398-025-03377-5
Age-dependent cortical overconnectivity in Shank3 mice is reversed by anesthesia
Abstract
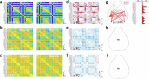
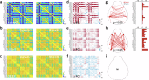
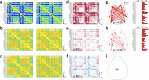
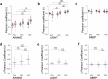
Growing evidence points to brain network dysfunction as a central neurobiological basis for autism spectrum disorders (ASDs). As a result, studies on Functional Connectivity (FC) have become pivotal for understanding the large-scale network alterations associated with ASD. Despite ASD being a neurodevelopmental disorder, and FC being significantly influenced by the brain state, existing FC studies in mouse models predominantly focus on adult subjects under anesthesia. The differential impact of anesthesia and age on cortical functional networks in ASD subjects remains unexplored. To fill this gap, we conducted a longitudinal evaluation of FC across three brain states and three ages in the Shank3b mouse model of autism. We utilized wide-field calcium imaging to monitor cortical activity in Shank3b+/- and Shank3b+/+ mice from late development (P45) through adulthood (P90), and isoflurane anesthesia to manipulate the brain state. Our findings reveal that network hyperconnectivity, emerging from the barrel-field cortices during the juvenile stage, progressively expands to encompass the entire dorsal cortex in adult Shank3b+/- mice. Notably, the severity of FC imbalance is highly dependent on the brain state: global network alterations are more pronounced in the awake state and are strongly reduced under anesthesia. These results underscore the crucial role of anesthesia in detecting autism-related FC alterations and identify a significant network of early cortical dysfunction associated with autism. This network represents a potential target for non-invasive translational treatments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Altered sex differences related to food intake, hedonic preference, and FosB/deltaFosB expression within central neural circuit involved in homeostatic and hedonic food intake regulation in Shank3B mouse model of autism spectrum disorder.Neurochem Int. 2024 Dec;181:105895. doi: 10.1016/j.neuint.2024.105895. Epub 2024 Oct 24. Neurochem Int. 2024. PMID: 39461669
-
Increasing isoflurane dose reduces homotopic correlation and functional segregation of brain networks in mice as revealed by resting-state fMRI.Sci Rep. 2018 Jul 12;8(1):10591. doi: 10.1038/s41598-018-28766-3. Sci Rep. 2018. PMID: 30002419 Free PMC article.
-
Juvenile Shank3b deficient mice present with behavioral phenotype relevant to autism spectrum disorder.Behav Brain Res. 2019 Jan 1;356:137-147. doi: 10.1016/j.bbr.2018.08.005. Epub 2018 Aug 19. Behav Brain Res. 2019. PMID: 30134148 Free PMC article.
-
Idiosyncratic organization of cortical networks in autism spectrum disorder.Neuroimage. 2019 Apr 15;190:182-190. doi: 10.1016/j.neuroimage.2018.01.022. Epub 2018 Jan 31. Neuroimage. 2019. PMID: 29355768
-
Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications.J Neurodev Disord. 2021 Nov 16;13(1):55. doi: 10.1186/s11689-021-09397-8. J Neurodev Disord. 2021. PMID: 34784886 Free PMC article. Review.
References
-
- Nakai N, Sato M, Yamashita O, Sekine Y, Fu X, Nakai J, et al. Virtual reality-based real-time imaging reveals abnormal cortical dynamics during behavioral transitions in a mouse model of autism. Cell Rep. 2023;42:112258. - PubMed
MeSH terms
Substances
Grants and funding
- PHEM, GSA22E006/Fondazione Telethon (Telethon Foundation)
- RICONSIN/Banca d'Italia (Bank of Italy)
- 2022YCTLPL/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- ECS_00000017 MUR_ PNRR/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

