Anion intercalation enables efficient and stable carboxylate upgrading via aqueous non-Kolbe electrolysis
- PMID: 40253422
- PMCID: PMC12009357
- DOI: 10.1038/s41467-025-58924-x
Anion intercalation enables efficient and stable carboxylate upgrading via aqueous non-Kolbe electrolysis
Abstract
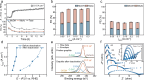
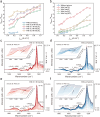
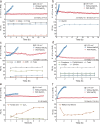
Next-generation techniques for sustainable carboxylate production generate carboxylate salts as the primary outcome. To circumvent the costly conversion of carboxylate salts to acids, we demonstrate the aqueous (non-)Kolbe electrolysis process as an alternative strategy to generate downstream value-added chemicals. Upon revealing the irreversible oxidation-induced charge transfer inhibition on the graphite anode, we propose an anion intercalation strategy to mitigate the stability problem induced by the ever-increasing overpotential. In acetate decarboxylation, we observe a high Faradaic efficiency of ~95% for non-Kolbe products (methanol and methyl acetate) at wide current densities ranging from 0.05 to 1 A cm-2 and long-term stability at current densities of 0.15 and 0.6 A cm-2 for 130 and 35 h, respectively. We also extended this strategy for the upgrading of long-chain carboxylates such as propionate, butyrate, and succinate. Our work provides valuable guidance for carboxylate upgrading and extendable strategy for overcoming passivation challenges in catalysis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A China provisional patent application (202510190131.9) based on the technology described in this work was filed on Mar. 2025 by C.X., X.Z., X.L., X.F., Q.J., and T.Z. at the University of Electronic Science and Technology of China. The other authors declare no competing interests.
Figures

References
-
- Zheng, T. et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol.16, 1386–1393 (2021). - PubMed
-
- Overa, S. et al. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. Nat. Catal.5, 738–745 (2022).
-
- Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science378, 207–211 (2022). - PubMed
-
- Zhou, H. et al. Selectively upgrading lignin derivatives to carboxylates through electrochemical oxidative C(OH)−C bond cleavage by a Mn-doped cobalt oxyhydroxide catalyst. Angew. Chem. Int. Ed.60, 8976 (2021). - PubMed
Grants and funding
- 22221003/National Natural Science Foundation of China (National Science Foundation of China)
- 22309171/National Natural Science Foundation of China (National Science Foundation of China)
- 52171201/National Natural Science Foundation of China (National Science Foundation of China)
- 2024SKL-A-01/State Key Laboratory of Catalysis (State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics)
LinkOut - more resources
Full Text Sources

